Chemistry students solve decades-old oxidation puzzle
Students at the University of Amsterdam have designed a new catalyst that can render important chemical processes more sustainable. Their catalyst can create selective peroxide-like reagents literally from thin air and uses those to oxidise alcohols to carbonyl compounds in a dual-action mechanism. The results have just been published online by Chemistry: A European Journal.
Thierry Slot, a master student at the Research Priority Area Sustainable Chemistry, has succeeded in solving a thorny problem in organic chemistry: the selective catalytic oxidation of activated alcohols with molecular oxygen (air). Working with second-year bachelor students Peter Jungbacker and Dylan van Noordenne, Slot designed and synthesised a dual-action solid catalyst that facilitates a cascade of oxygen activation followed by alcohol dehydrogenation.
The new catalyst is made mostly of carbon, with a sprinkling of nitrogen, oxygen, and cobalt, iron or copper. Importantly, it contains no noble metals such as platinum or palladium. Replacing rare and costly noble metals with catalysts based on first-row transition metals is a key theme of the UvA's Research Priority Area Sustainable Chemistry.
Benzylic and allylic alcohols are important intermediates in fine-chemical synthesis, as well as in the agrochemicals and flavours and fragrances sectors. Oxidising these alcohols to aldehydes and ketones is tricky, because other parts of the molecule can also be oxidised along the way. This is especially true if you use molecular oxygen or air, because activating oxygen requires high temperatures, which can set off side reactions. There are two ways around this problem: use a platinum catalyst, or use an activated oxidant such as a peroxide molecule. But platinum is extremely rare and expensive, and peroxides are hazardous reagents, and also more expensive than air.
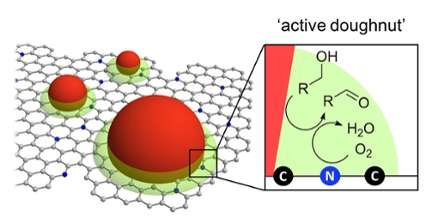
The Sustainable Chemistry team designed a new catalyst based on a new type of nitrogen-doped carbon that was developed in the group a few months ago. This material can "donate" electrons to oxygen molecules, lengthening the O–O bond and creating a sort of "peroxide" literally from thin air. Here and there on the active surface, the team placed metal oxide nanoparticles that can catalyse the organic oxidation of alcohols. This combination creates doughnut-shaped zones around the particles where both the oxygen activation and alcohol oxidation can occur (see figure above). Indeed, this "active doughnut" concept has implications for several other bifunctional solid catalysts.
The project was designed and supervised by Prof. Gadi Rothenberg and Dr. David Eisenberg. Rothenberg has a long history with this reaction: "My PhD project focused on allylic and benzylic oxidation catalysis. We tried working with oxygen, but the selectivity was always low, so at the end I ran most of my experiments with peroxide reagents. Now, twenty years later, we've finally solved the problem using these special materials that can activate oxygen from the air selectively under mild conditions".
The results have been published in Chem. Eur. J. as a communication. Slot is now working on expanding the concept to other oxidation reactions, in collaboration with exchange students from the Holland Research School of Molecular Chemistry (HRSMC) excellence master program.
More information: Thierry K. Slot et al. Cooperative catalysis for selective alcohol oxidation with molecular oxygen, Chemistry - A European Journal (2016). DOI: 10.1002/chem.201602964
Journal information: Chemistry – A European Journal
Provided by University of Amsterdam



















