July 25, 2016 report
Aluminum-based electrochemical cell captures and sequesters carbon emissions and generates electricity
(Phys.org)—A pair of researchers at Cornell University has created an aluminum-based electrochemical cell that captures and sequesters carbon emissions while simultaneously generating a large amount of electricity. In their paper published in the journal Science Advances, Wajdi Al Sadat and Lynden Archer describe the cell, how it works and why they believe it is better than other carbon-capturing cells that have been developed to date.
Human beings have been pumping carbon dioxide into the atmosphere in large amounts since the advent of the industrial age, leading to global warming. Scientists have been working overtime to figure out a way to reduce the amounts of the gas that we emit, but while there have been great improvements, far too much is still going into the air. Meanwhile, other scientists are taking a different approach—they are looking for ways to remove carbon dioxide from the atmosphere in an economically feasible way. In this new effort, the researchers at Cornell report on cell technology they developed that differs significantly from the approach used by others.
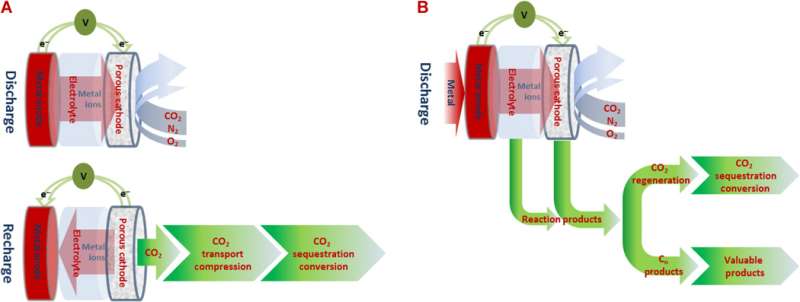
To create a cell that removes carbon dioxide, researchers have tried using magnesium, lithium or sodium as an anode—the results to date have worked to some extent, but they produce carbonates, which are not very useful. To get around that problem, the researchers instead used aluminum foil to make the anode—the cathode was made of stainless steel mesh and an ionic liquid with some aluminum chloride salt in it was used as the electrolyte.
In testing their cell, the duo found that it was capable of generating up to 13 ampere-hours for every gram of carbon that it captured. They point out that it did so without the need for any sort of catalyst and that it was done at room temperature. In addition, the output from the system consisted of aluminum oxalate, which they noted can be used to make oxalic acid, a material that is commonly used in several industries, which means it could be easily sold.
The researchers acknowledge that their cell has one serious drawback at the moment—it won't work if water is present in the mix of gasses it pulls in, a situation that would exist in most real-world locations. They plan to search for another electrolyte that is less sensitive to moisture to solve that problem.
More information: The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation, Science Advances 20 Jul 2016: Vol. 2, no. 7, e1600968. DOI: 10.1126/sciadv.1600968
Abstract
Economical and efficient carbon capture, utilization, and sequestration technologies are a requirement for successful implementation of global action plans to reduce carbon emissions and to mitigate climate change. These technologies are also essential for longer-term use of fossil fuels while reducing the associated carbon footprint. We demonstrate an O2-assisted Al/CO2 electrochemical cell as a new approach to sequester CO2 emissions and, at the same time, to generate substantial amounts of electrical energy. We report on the fundamental principles that guide operations of these cells using multiple intrusive electrochemical and physical analytical methods, including chronopotentiometry, cyclic voltammetry, direct analysis in real-time mass spectrometry, energy-dispersive x-ray spectroscopy, x-ray photoelectron spectroscopy, and coupled thermogravimetric analysis–Fourier transform infrared spectroscopy. On this basis, we demonstrate that an electrochemical cell that uses metallic aluminum as anode and a carbon dioxide/oxygen gas mixture as the active material in the cathode provides a path toward electrochemical generation of a valuable (C2) species and electrical energy. Specifically, we show that the cell first reduces O2 at the cathode to form superoxide intermediates. Chemical reaction of the superoxide with CO2 sequesters the CO2 in the form of aluminum oxalate, Al2(C2O4)3, as the dominant product. On the basis of an analysis of the overall CO2 footprint, which considers emissions associated with the production of the aluminum anode and the CO2 captured/abated by the Al/CO2-O2 electrochemical cell, we conclude that the proposed process offers an important strategy for net reduction of CO2 emissions.
Journal information: Science Advances
© 2016 Phys.org