How water droplets freeze: The physics of ice and snow

Freezing water is a central issue for climate, geology and life. On earth, ice and snow cover 10 percent of the land and up to half of the northern hemisphere in winter. Polar ice caps reflect up to 90 percent of the sun's incoming radiation. But how water droplets freeze, whether from within or from the surface, has been a topic of much controversy over past decade among chemists and physicists.
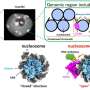
A team of researchers at Beijing Institute of Technology and Zhejiang University in China propose another question, "Where in the droplet does the crystallization of water or liquid silicon begin?" The team explains their findings this week in The Journal of Chemical Physics. This is an interesting problem and one that is crucial to understanding the crystallization mechanism of nanoscale tetrahedral liquid drops like water and silicon.
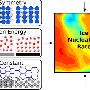
In their work, they used computer simulation, to find that the ripple-like density waves are markedly excited before crystallization of liquid silicon drops and films due to the volume expansion in a confined environment. The ripple-like density fluctuations create waves capable of promoting nucleation, eventually resulting in a ripple-like distribution of nucleation probability in drops and films. These results suggest that the freezing of nanoscale water or silicon liquid drops is initiated at a number of different distances from the center of the droplet, providing new insights on a long-standing dispute in the field of material and chemical physics.
The research team employed a molecular dynamics simulation to investigate the freezing of nanoscale silicon drops and films, a method widely used for the investigations of microscopic thermodynamic and dynamic process. In computer simulations of crystallization events, the short simulation time makes it difficult to observe. To address this issue some special simulation methods, namely, the rare event sampling algorithms, were proposed. But these methods inevitably drop some high probability regions of nucleation in the trajectory sampling starting from a single configuration, so the team employed brute-force simulation and sampled massive and independent crystallization processes. "Although the method is 'brute,' it can faithfully represent the distribution of nuclei," explained Yongjun Lü, a physicist at Beijing Institute of Technology and Zhejiang University. "This is why we were able to observe the ripple-like distribution of nucleation probability while it is absent in other studies."
A challenge for the team was the great calculation costs. To achieve the credible probability distributions of nucleation in drops and films requires massive statistical sampling, requiring more than 6 months of CPU time.
The implications of this research are far-reaching. "We can extend the present results to all the tetrahedral liquids including water due to their similarity in molecular structure," Lü said. "It suggests that the surface environment does not play a decisive role in the formation of ice and snow as expected. The density fluctuations inside drops result in that the possible freezing regions cover the middle and the surface regions, depending on the drop size. The freezing from the surface or from within may be random."
The next steps for the research team are to simulate the crystallization of water in geometric constrained space and under high-pressure conditions to study the freezing mechanism of water in microcracks and micropores of rock. By deepening the understanding of the freezing of water drops will significantly enhance the understanding of its effects on the planet and its climate.
More information: "Density-wave-modulated crystallization in nanoscale silicon films and droplets," Yongjun Lü, Qingling Bi and Xinqing Yan, Journal of Chemical Physics , June 21, 2016. DOI: 10.1063/1.4953038
Journal information: Journal of Chemical Physics
Provided by American Institute of Physics