Biofuel researchers employ Titan to probe 'lignin shield'
When the Ford Motor Company's first automobile, the Model T, debuted in 1908, it ran on a corn-derived biofuel called ethanol, a substance Henry Ford dubbed "the fuel of the future."
Cheap fossil fuels undermined Ford's vision of wide-scale renewable transportation fuel, but recent advancements in biofuel research, including research conducted at the US Department of Energy's (DOE's)Oak Ridge National Laboratory (ORNL) and BioEnergy Science Center (BESC), have rekindled the dream of economically viable ethanol.
Today, biofuel research is focused on cellulosic ethanol—alcohol made from woody plants and waste biomass, such as corn stalks and leaves. Currently, breaking down these plant materials into simple sugars is a costly and complex process, requiring large quantities of acid, water, and heat. Experimental pretreatments, however, hold the promise of driving down these costs by making more of the biomass available for fermentation by microbes.
Furthermore, researchers are using high-performance computing resources, including the Titan supercomputer at ORNL, to gain a better understanding of why these experimental pretreatments work—a measure that helps scientists refine effective techniques and develop new ones.
Recently, a team led by Jeremy Smith, a Governor's Chair at the University of Tennessee (UT) and the director of the UT–ORNL Center for Molecular Biophysics (CMB), used the Oak Ridge Leadership Computing Facility's(OLCF's) Titan supercomputer at ORNL to gain insight into the effectiveness of an experimental pretreatment developed by BESC researchers in California called Cosolvent Enhanced Lignocellulose Fractionation, or CELF. The OLCF is a DOE Office of Science User Facility located at ORNL.
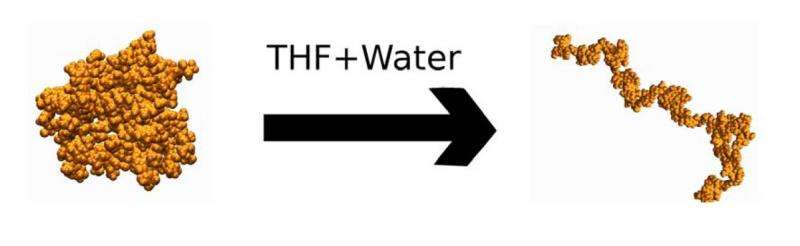
Recent studies have shown CELF to be more than three times as effective as conventional dilute acid pretreatment in maple wood. To understand why, the team simulated lignin, a problematic molecule for biofuel production, in a two-component CELF solvent consisting of water and tetrahydrofuran (THF), an industrial chemical commonly used in polyvinyl chloride, or PVC, manufacturing and varnish. The simulation results, published in Green Chemistry, suggest that THF, which binds favorably with both water and lignin, acts as a barrier between the two, making the undesired, water-repelling lignin easier to remove.
The study is important because lignin, a critical component of plants' defense system against predators and disease, poses one of the biggest hurdles to cost-effective ethanol production. Developing new chemical pretreatments, like the THF–water cosolvent, and finding out why they work could provide insights that neutralize the problem.
"With conventional pretreatment, the simulations showed that lignin clumps up because it wants to limit its interaction with water. It aggregates and binds to cellulose, the substance that is converted into ethanol, and poses a physical barrier for the enzymes, preventing them from reaching cellulose," said ORNL staff scientist Loukas Petridis.
"In the presence of THF, however, lignin opens into long coils, which can be more easily removed when the biomass is washed. Furthermore, our simulation showed that THF prefers to solvate close to the surface of lignin, meaning it likes to interact with the molecule. This helps explain why CELF is good for removing lignin—THF acts like a shield protecting lignin from water."
Solvating with Supercomputers
Jeremy Smith's team constructed its 250,000-atom model under an allocation on Titan, awarded through DOE'sOffice of Advanced Scientific Computing Research (ASCR) Leadership Computing Challenge, or ALCC, program. The model, built using a molecular dynamics code called GROMACS, consisted of a 61-unit lignin polymer submerged in a 2.1-nanometer cube of the THF–water cosolvent.
GROMACS calculated the motion of the lignin–cosolvent system in time steps of 2 femtoseconds, or 2,000 trillionths of a second. At this timescale, researchers could comfortably obtain 40–50 nanoseconds of simulation time per day on Titan, a Cray XK7 with a peak performance of 27 petaflops (or 27 quadrillion calculations per second).
"That's about 10 times faster than we could run otherwise," said team member Micholas Smith, a CMB postdoctoral researcher.
The team ran the simulation for 200 nanoseconds, storing the resulting 18 terabytes of data in the OLCF's High-Performance Storage System.
To see how lignin responds under slightly different conditions, the team tested the lignin–cosolvent system using three different THF–water solvent ratios and four different temperatures, mirroring conditions carried out in experiment. Results indicated that the cosolvent was just as effective in low temperatures as it was in high temperatures.
"That's important because high temperatures are an expensive part of pretreatment," Petridis said. "Biofuel engineers could lower the pretreatment temperature and know that it would not be detrimental to CELF."
Although THF excludes much of the water from reaching lignin, the ORNL team found that it also traps water near lignin sites that are easily broken by acid. "This may indicate that THF helps the pretreatment process in other ways," Micholas Smith said.
"One way is that it may make the sites more available for the acid to access and break the bonds. On top of that, THF may help facilitate the chemical reaction that cuts lignin loose. If we could work out the mechanism by which it breaks apart, maybe we could come up with a catalyst to help that."
Testing such a hypothesis requires the simulation of chemical reactions and chemical bonding, a computationally demanding task that depends on a different kind of molecular dynamics code capable of accounting for the subatomic interactions of the lignin–cosolvent system.
"Our current simulation confirms that THF facilitates lignin bond-breaking," Micholas Smith said. "In the future, we hope to take the next step and explore how that process works in greater detail."
Future simulations of biomass, lignin, and pretreatment processes is being carried out on Titan under a 100-million core-hour allocation awarded to Jeremy Smith's team as part of the 2016 Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program.
The research was funded by the BioEnergy Science Center, a DOE Bioenergy Research Center supported by DOE's Office of Science.
More information: Micholas Dean Smith, Barmak Mostofian, Xiaolin Cheng, Loukas Petridis, Charles M. Cai, Charles E. Wyman, and Jeremy C. Smith, "Cosolvent pretreatment in cellulosic biofuel production: Effect of tetrahydrofuran-water on lignin structure and dynamics." Green Chemistry (2016), DOI: 10.1039/C5GC01952D
Journal information: Green Chemistry
Provided by Oak Ridge National Laboratory