Neanderthal genes gave modern humans an immunity boost, allergies
When modern humans met Neanderthals in Europe and the two species began interbreeding many thousands of years ago, the exchange left humans with gene variations that have increased the ability of those who carry them to ward off infection. This inheritance from Neanderthals may have also left some people more prone to allergies.
The discoveries reported in two independent studies in the American Journal of Human Genetics on January 7 add to evidence for an important role for interspecies relations in human evolution and specifically in the evolution of the innate immune system, which serves as the body's first line of defense against infection.
"We found that interbreeding with archaic humans—the Neanderthals and Denisovans—has influenced the genetic diversity in present-day genomes at three innate immunity genes belonging to the human Toll-like-receptor family," says Janet Kelso of the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany.
"These, and other, innate immunity genes present higher levels of Neanderthal ancestry than the remainder of the coding genome," adds Lluis Quintana-Murci of the Institut Pasteur and the CNRS in Paris. "This highlights how important introgression events [the movement of genes across species] may have been in the evolution of the innate immunity system in humans."
Earlier studies have shown that one to six percent of modern Eurasian genomes were inherited from ancient hominins, such as Neanderthal or Denisovans. Both new studies highlight the functional importance of this inheritance on Toll-like receptor (TLR) genes—TLR1, TLR6, and TLR10. These TLR genes are expressed on the cell surface, where they detect and respond to components of bacteria, fungi, and parasites. These immune receptors are essential for eliciting inflammatory and anti-microbial responses and for activating an adaptive immune response.
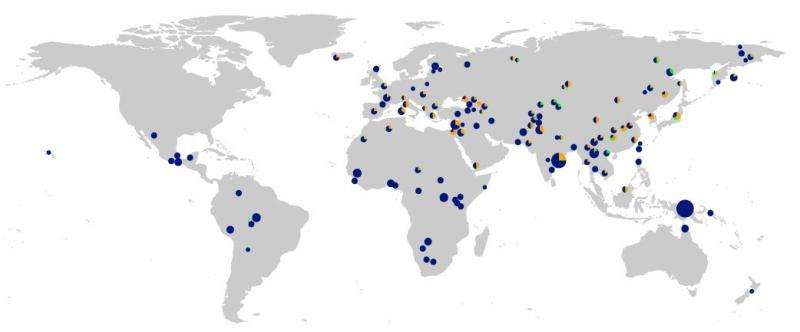
Quintana-Murci and his colleagues set out to explore the evolution of the innate immune system over time. They relied on vast amounts of data available on present-day people from the 1000 Genomes Project together with the genome sequences of ancient hominins. Quintana-Murci's team focused on a list of 1,500 genes known to play a role in the innate immune system. They then examined patterns of genetic variation and evolutionary change in those regions relative to the rest of the genome at an unprecedented level of detail. Finally, they estimated the timing of the changes in innate immunity and the extent to which variation in those genes had been passed down from Neanderthals.
These investigations revealed little change over long periods of time for some innate-immunity genes, providing evidence of strong constraints. Other genes have undergone selective sweeps in which a new variant came along and quickly rose to prominence, perhaps because of a shift in the environment or as a result of a disease epidemic. Most adaptations in protein-coding genes occurred in the last 6,000 to 13,000 years, as human populations shifted from hunting and gathering to farming, they report.
But, Quintana-Murci says, the biggest surprise for them "was to find that the TLR1-6-10 cluster is among the genes presenting the highest Neanderthal ancestry in both Europeans and Asians."
Kelso and her colleagues came to the same conclusion, but they didn't set out to study the immune system. Their interest was in understanding the functional importance of genes inherited from archaic humans more broadly. They screened present-day human genomes for evidence of extended regions with high similarity to the Neanderthal and Denisovan genomes,then examined the prevalence of those regions in people from around the world. Those analyses led them to the same three TLR genes.
Two of those gene variants are most similar to the Neanderthal genome, whereas the third is most similar to the Denisovan genome, Kelso's group reports. Her team also provides evidence that these gene variants offered a selective advantage. The archaic-like variants are associated with an increase in the activity of the TLR genes and with greater reactivity to pathogens. Although this greater sensitivity might protect against infection, it might also increase the susceptibility of modern-day people to allergies.
"What has emerged from our study as well as from other work on introgression is that interbreeding with archaic humans does indeed have functional implications for modern humans, and that the most obvious consequences have been in shaping our adaptation to our environment - improving how we resist pathogens and metabolize novel foods," Kelso says.
As surprising as it may seem, it does make a lot of sense, she adds. "Neanderthals, for example, had lived in Europe and Western Asia for around 200,000 years before the arrival of modern humans. They were likely well adapted to the local climate, foods, and pathogens. By interbreeding with these archaic humans, we modern humans gained these advantageous adaptations."
More information: American Journal of Human Genetics, Deschamps et al.: "Genomic Signatures of Selective Pressures and Introgression from Archaic Hominins at Human Innate Immunity Genes" dx.doi.org/10.1016/j.ajhg.2015.11.014
This work was primarily supported by the Institut Pasteur, the Centre Nationale de la Recherche Scientifique (CNRS), and the Agence Nationale de la Recherche.
American Journal of Human Genetics, Dannemann et al.: "Introgression of Neandertal- and Denisovan-like Haplotypes Contributes to Adaptive Variation in Human Toll-like Receptors" dx.doi.org/10.1016/j.ajhg.2015.11.015
Journal information: American Journal of Human Genetics
Provided by Cell Press