The hidden danger of heavy metals in catalytic converters

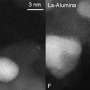
The heart of every car is its engine, where the energy of petroleum combustion is transformed to mechanical energy, which makes the car move. Unfortunately, no internal combustion engines are environmentally friendly. Carbon monoxide, nitrogen oxides, hydrocarbons and other dangerous compounds are released as exhaust. In order to protect the environment from these toxic gases, every modern car is equipped with a catalytic converter, which converts exhaustive gases to non-toxic water, nitrogen and carbon dioxide. Its function is based on the catalytic reaction, which takes place on the surface of precious metals—namely palladium, platinum and rhodium.
But such autocatalysts turn out to have a dangerous drawback and can create a huge problem in the future: Particles of the metals can be leached from the catalyst by the gas flow, leading to the contamination of the atmosphere, soil and surface water by heavy metals (Pt, Pd, Rh).
Indeed, every modern car equipped with autocatalysts may release toxic heavy metals during engine operation. This fact hasn't been widely discussed and remains underestimated.
The present study was conducted by high school student Gleb Rukhovich under the supervision of Ph.D. student Leonid Romashov in the laboratory of Prof. Valentine Ananikov at Zelinsky Institute, Moscow. They demonstrated that contact with water facilitates simple platinum and palladium salts to aggregate into various clusters—chemical species, which contain more than one metal atom. It is an important observation, since the toxicity of clusters can significantly exceed the toxicity of simple salts. Therefore, the eco-toxicological danger of environmental pollution by heavy metals should become a crucial research subject.
More information: Leonid V. Romashov et al. Analysis of model Pd- and Pt-containing contaminants in aqueous media using ESI-MS and the fragment partitioning approach, RSC Adv. (2015). DOI: 10.1039/c5ra22257e
Provided by Zelinsky Institute of Organic Chemistry