Improving catalysis through nanoconcentrator systems
A group of scientists at the University of Amsterdam (UvA) has developed a new approach in enhancing catalytic performance. In the current issue of Nature Chemistry they present functionalised, self-assembled nanospheres that enable highly efficient catalytic conversion by acting as 'nanocentrators'.
The new catalytic nanosphere concept was inspired by the working principles of natural enzymes. These bind molecules in well-defined pockets close to their active sites, thus introducing a pre-organization organisation that facilitates highly efficient transformations. The researchers mimic this enzymatic behaviour in synthetic nanocontainers that in addition, which can contain very high local catalyst concentrations , which and further enhances the catalytic performance.
Self-assembly
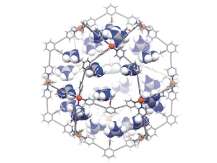
The new nanocontainers are formed by self-assembly: mixing 12 palladium metals and 24 so-called ditopic nitrogen ligands leads to formation of nano-sized spheres. The ligands are modified with guanidinium binding motifs so that the resulting nanocontainers are able to bind sulfonates and carboxylates in their interior. Sulfonate guests are thereby bound much more strongly than carboxylates because of so-called cooperative binding (employing multiple binding sites). The researchers use this to firmly fix the sulfonated gold-based catalyst, while the remaining binding sites are available for the pre-organisation of the carboxylate moieties that are to be converted (the substrates).
Enhanced reaction rates
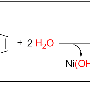
The working principles of this 'nanoconcentrator' system were established using a gold-catalysed cyclization reaction (shown above). The local high concentration of the metal catalyst combined with the pre-organization of the substrate resulted in dramatically enhanced reaction rates in comparison to common systems where the catalyst and the reactants are not pre-organised but just both dissolved in a solvent. Reaction rates usually increase with catalyst and substrate concentration; however this is generally limited by solubility issues or unfavorable catalyst/reactant ratios. This issue has now been solved by taking advantage of local concentrations in the self-assembled nanoconcentrator.
Widely applicable strategy
Since many existing metal catalysts are utilized with sulfonate groups (generally to make them water soluble), the presented nanoconcentrator system potentially provides a widely applicable general strategy to many different reactions. Furthermore, the researchers established that the encapsulated sulfonate-containing gold catalysts did not (or only slowly) convert neutral (acid) substrates. This provides a starting point for the development of more complex catalyst systems with substrate-selective catalysis and base-triggered on/off switching.
More information: Qi-Qiang Wang et al. Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions, Nature Chemistry (2016). DOI: 10.1038/nchem.2425
Journal information: Nature Chemistry
Provided by University of Amsterdam