Study sheds light on aluminum reaction in low pH environments
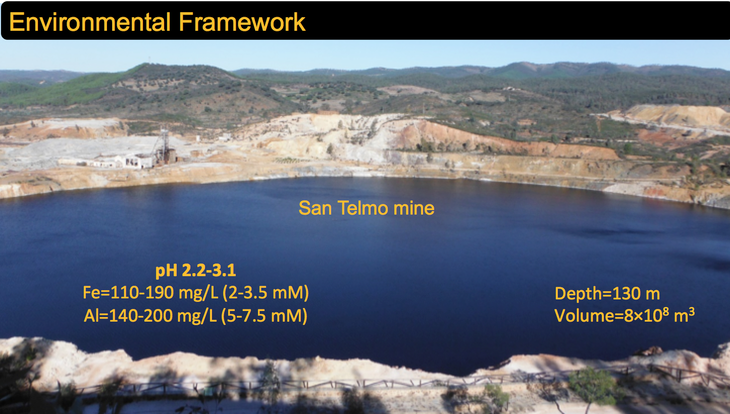
Within the deep strata of the San Telmo "acid pit lake" in southwestern Spain rests a wealth of rich geochemical knowledge. That knowledge recently helped researchers at Penn State discover new information about the chemical reactions of aluminum in low pH environments.
San Telmo is one of the larger pit lakes within the Iberian Pyrite Belt, which stretches along the southern border of the Iberian Peninsula. Numerous pit lakes have formed along this 150-mile stretch of land as a result of former open cast mining operations for precious metals.
When the mining pits were abandoned, they became flooded with rainwater and groundwater, which then reacted with remaining rocks and resulted in highly acidic water that had high concentrations of metals. Although an environmental hazard, these acidic lakes provide exceptional natural laboratories for the study of geochemical and biogeochemical processes under extreme conditions.
Javier Sánchez-España, senior scientist at the Geological and Mining Institute of Madrid, Spain; Iñaki Yusta, professor of mineralogy at the University of Basque Country, Bilbao, Spain; William Burgos, professor of environmental engineering at Penn State; and Jennifer Gray, research associate at the Materials Research Institute at Penn State, utilized the extreme conditions of San Telmo to better understand the exact pH in which aluminum precipitates out of water. The results gleaned from this study will help researchers find more successful techniques for removing the toxic metal from natural water resources near mining areas and from the sludge generated at waste treatment facilities.
Their study was recently published in the research journal Geochimica et Cosmochimica Acta.
"The behavior of aluminum in acidic systems is still the subject of intense scientific debate," Sánchez-España said. "Dissolved aluminum ions are highly toxic for most aquatic microorganisms, so the geochemistry of this metal is of concern for many researchers and water quality protection agencies."
The researchers began their study by finding and mapping out an ideal location in the lake, one with a pH between 2.5-3.0. They then took samples of the water using Van Dorn-type water samplers for open water and several sondes, devices that can measure multiple parameters at once.
"We took geochemical profiles of many parameters—pH, redox potential, temperature, conductivity, dissolved oxygen—and many dissolved chemical elements," Sánchez-España said.
On average, the researchers obtained five to 10 profiles per sampling season, with three to four sampling seasons per year, during a five-year period.
They then used the samples to build geochemical depth profiles in the lake and track major and minor elements.
The team expected to see certain trends based on the pH levels, but given the extremely low pH values in San Telmo, it did not expect to see aluminum precipitating out of the water.
"The general conceptualization is that iron drops out first because it will precipitate at lower pH values and then aluminum will follow," Burgos said. "For example, if you were moving down a stream, you would see the stream stained orange first because the iron drops out and then you would see it stained white because the aluminum drops out, so you have this sort of spatial separation of these metal precipitates, typically based on pH."
At San Telmo, however, the researchers were seeing white precipitates in a region where they should have been seeing orange precipitates and so the observation that aluminum was being removed when iron was being removed was an anomaly.
This irregularity prompted the research team to find a way to calculate exactly when the aluminum was precipitating out of the water, but this wasn't an easy task.
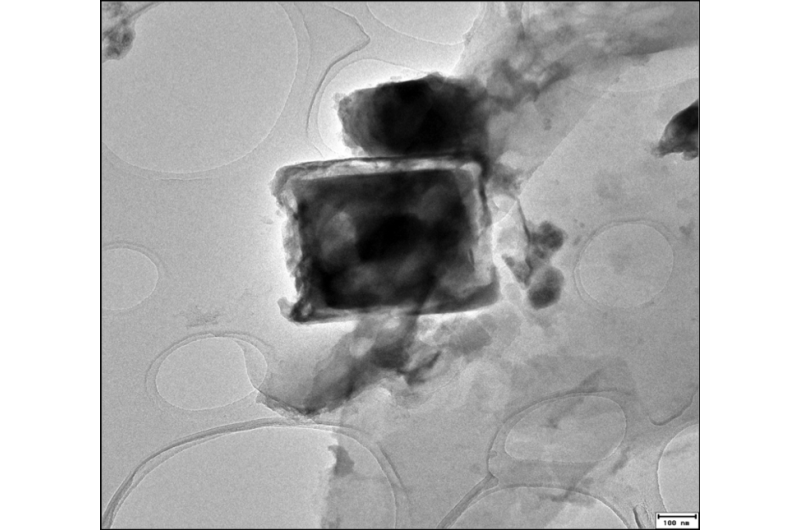
Macroscopically, they could see the aluminum disappearing from the water, but the particles were too small to identify using traditional microscopes; therefore, Sánchez-España obtained a Fulbright Scholarship in order to bring the samples to Penn State so he could utilize the powerful imaging capabilities of the Titan3 scanning transmission electron microscope, one of the world's most powerful microscopes, at the Materials Research Institute.
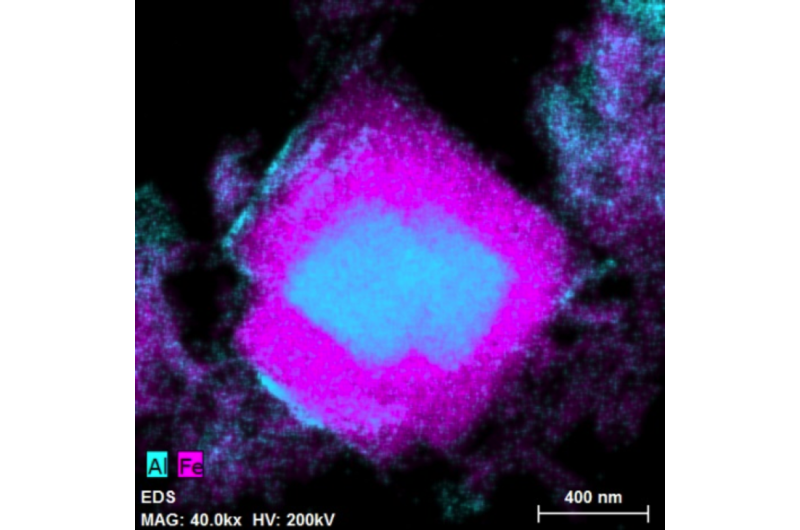
"We obtained hundreds of high-resolution images, which nicely show the distribution of many different chemical elements, in addition to crystallographic parameters of many aluminum crystals," Sánchez-España said. "This information has allowed us to significantly improve our degree of knowledge on the behavior of aluminum at low pH levels."
The Titan3 also allowed the researchers to determine what was causing the aluminum to precipitate out of the water at the same time as the iron.
Previously, the observation was that there was a "miscibility gap" between the formation of aluminum-containing iron solids and iron-containing aluminum solids. Specifically, the complete mixing of the aluminum mineral alunite and the iron mineral jarosite had never been reported before.
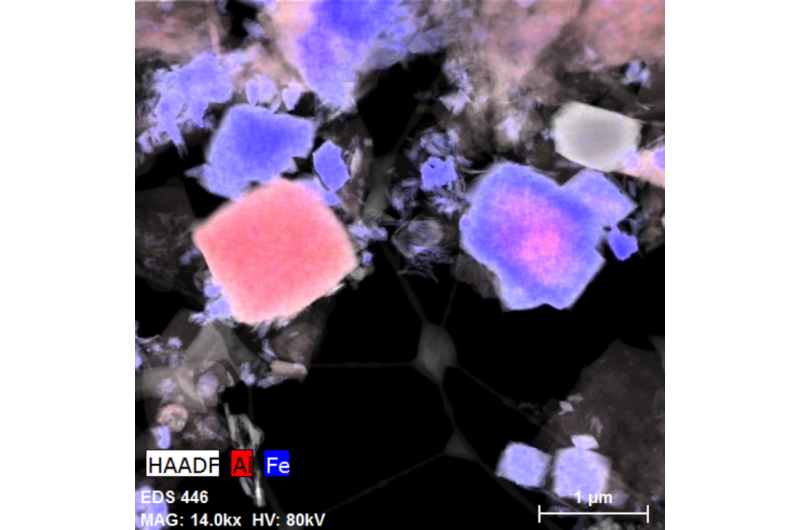
"You don't go from 100 percent and zero percent to 50/50," Burgos explained. "Instead, researchers saw a lot of iron-substituted alunite or aluminum-substituted jarosite."
But the Titan3 images revealed that the miscibility gap doesn't actually exist.
"We saw that the two elements were completely overgrowing one another in the middle," Burgos said. "So the solid solution miscibility gap is spanned right in that little nanoparticle and nobody's seen that before."
Their investigations showed that a wide variety of solids might be formed under these conditions.
"The geochemistry of aluminum is strongly linked with that of iron, more than we have considered before," Sánchez-España said. "I think this finding will have important implications in the future in regard to the study of the mobility of this metal in the environment. Aluminum may be removed from a solution via a 'macroscopically invisible' pathway, not as a specific solid phase, but as adsorbed ions present in the surfaces of iron mineral phases and/or as impurities in the crystalline structure of the later."
The next phase of their study will focus on two smaller pit lakes in the same area, the Guadiana pit lake in Herrerías mine and Cueva de la Mora pit lake. These two lakes have slightly higher pH levels (around 4.0-4.5) and therefore will allow a different suite of minerals to precipitate.
"At these conditions, many additional toxic metals may be significantly adsorbed on the aluminum phases," Sánchez-España said. "So the fate and transport of aluminum in these waters can also affect to the dynamics of many other pollutants."
-
View from above San Telmo "acid pit lake." Credit: Penn State -

The FEI Titan3 is the world's most powerful sub-Angstrom scanning transmission electron microscope. Located in a vibration and radiation shielded lab in the basement of the Millennium Science Complex, the Titan can see individual atoms and provide both chemical and elemental sample information. Credit: Penn State
More information: Javier Sánchez-España et al. Geochemistry of dissolved aluminum at low pH: Extent and significance of Al-Fe(III) coprecipitation below pH 4.0, Geochimica et Cosmochimica Acta (2015). DOI: 10.1016/j.gca.2015.10.035
Journal information: Geochimica et Cosmochimica Acta
Provided by Pennsylvania State University



















