Scientists prepare elusive organocatalysts for drug and fine chemical synthesis
Rice University scientists using an efficient metal-free process have synthesized dozens of small-molecule catalysts, tools that promise to speed the making of novel chemicals, including drugs.
The lab of synthetic chemist László Kürti made elusive chiral biaryl compounds in a single-flask process that does not require the use of transition metals.
These biaryls are called organocatalysts because they catalyze chemical reactions without metal ions. They eliminate the need for transition metals and simplify chemical processes to synthesize new molecules. Transition metals are conductive metals that include titanium, iron, nickel, silver, copper, palladium and gold and are commonly used in catalysis.
The new tools detailed in the Angewandte Chemie international edition open avenues for faster and more cost-effective chemical synthesis, Kürti said.
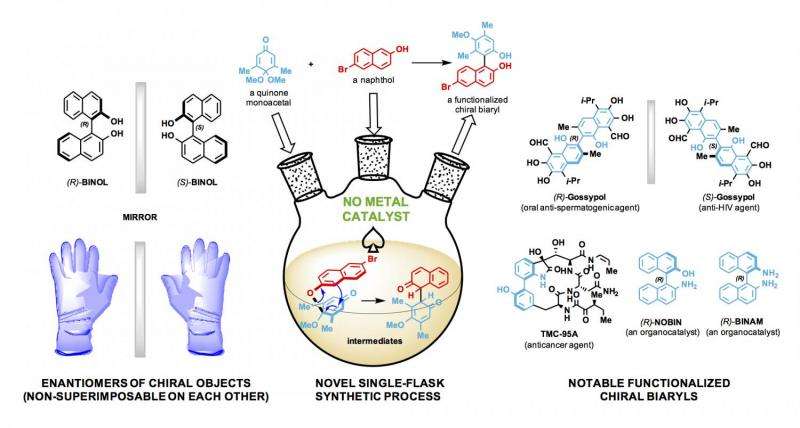
Biaryls are molecular compounds of two aromatic rings directly joined by a carbon-carbon bond. When functionalized, or altered, these biaryls (phenyl-phenyl, naphthyl-phenyl, thienyl-naphthyl and more) become highly selective, reliable and customizable catalysts, Kürti said.
Kürti's research uses biaryls as catalysts to develop novel single-enantiomer compounds.
Enantiomers are asymmetrical molecules found among organic compounds. Like one's hands, their structures are mirror images that cannot be superimposed on each other. Significantly, these twins can have radically different effects – one beneficial, one not—as they interact with enzymes, proteins, receptors and even other chiral catalysts. Pharmaceutical companies understandably want to make drugs that contain only the helpful enantiomer.
Currently, single-enantiomer compounds are painstakingly synthesized as building blocks for drugs, agricultural products and functional materials.
Kürti said that by decade's end, 95 percent of chiral drugs will be sold as single enantiomers. But synthesizing one particular enantiomer with precision and high efficiency is hard, especially via trial-and-error approaches that to now often require transition metal catalysts.
"For enantiomer preparations, you need catalysts," he said, but transition metals are expensive and can leave toxic residues that need to be removed before the compound can be used in clinical trials. The Rice lab's simple, cost-effective way to make chiral-functionalized biaryls not only eliminates the need for transition metals but can replace many steps in the synthesis process. Each step can take days or weeks.
"That's the basis of what we do: Develop new methods," Kürti said. "Part of my program is coming up with new catalyst structures. When we were looking at the various ways to put such compounds together, we stumbled upon this very interesting reaction."
The lab combined readily available compounds, including quinone monoacetal and naphthol, to make functionalized biaryls. "This is a major advance," he said. "Using these building blocks, we made 41 different chiral biaryl compounds in a relatively short time.
"The functionalized chiral biaryls are really versatile compounds. You can use them outright as organocatalysts or complex them with transition metals to make new transition-metal catalysts. So the possibilities are unlimited. Moreover, these compounds can be used as building blocks en route to natural products with biaryl substructures in them."
He said the biaryls "open up an entire world of new chemical space," as they lower the barrier to inventing and making new chemical compounds.
"The implications are huge," Kürti said. "This will certainly find its way into drug discovery, making agrochemicals and many other fine chemicals."
More information: Hongyin Gao et al. Practical Organocatalytic Synthesis of Functionalized Non- -Symmetrical Atropisomeric Biaryls , Angewandte Chemie International Edition (2015). DOI: 10.1002/anie.201508419
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Rice University












.jpg)