ORNL microscopy captures real-time view of evolving fuel cell catalysts
Atomic-level imaging of catalysts by scientists at the Department of Energy's Oak Ridge National Laboratory could help manufacturers lower the cost and improve the performance of emission-free fuel cell technologies.
Fuel cells rely on costly platinum catalysts to enable the reactions that convert chemical energy into electricity. Alloying platinum with noble metals such as cobalt reduces the overall cost, but such alloyed catalysts vary in performance based on their atomic structure and processing history.
An ORNL team used scanning transmission electron microscopy to track atomic reconfigurations in individual platinum-cobalt nanoparticle catalysts as the particles were heated inside the microscope. The in-situ measurements—acquired in real time in the vacuum of the microscope column—allowed the researchers to collect atomic level data that could not be obtained with conventional microscopy techniques. The results are published in Nature Communications.
"This is the first time individual nanoparticles have been tracked this way—to image the structural and compositional changes at the atomic level from the start of an annealing process to the finish," ORNL coauthor Karren More said.
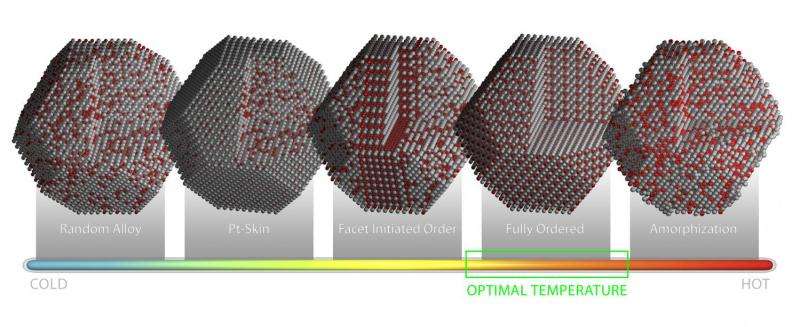
Very small changes in the positions of platinum and cobalt atoms affect the catalyst's overall activity and selectivity, so annealing—a gradual heating, holding, and cooling process—is often used to modify the alloy's surface structure. The ORNL in situ microscopy experiments documented exactly what, when and how specific atomic configurations originate and evolve during the annealing process.
"You can anneal something from room temperature to 800 degrees Celsius, but you don't know at which point you should stop the process to ensure the best catalytic performance," lead author Miaofang Chi said. "Because you don't know how the particle evolves, you might be missing the optimum surface configuration."
The atomic-level detail in the ORNL study will guide researchers and manufacturers who want to fine-tune their catalysts' atomic structure to meet the demands of a specific application.
"This work paves the way towards designing catalysts through post-synthesis annealing for optimized performance," Chi said.
The study is published as "Surface faceting and elemental diffusion behavior at atomic scale for alloy nanoparticles during in situ annealing."
More information: Miaofang Chi et al. Surface faceting and elemental diffusion behaviour at atomic scale for alloy nanoparticles during in situ annealing, Nature Communications (2015). DOI: 10.1038/ncomms9925
Journal information: Nature Communications
Provided by Oak Ridge National Laboratory