Better catalysts will remove carcinogenic chlorine compounds from water

The Institute of Physical Chemistry of the Polish Academy of Sciences in Warsaw has just unveiled two new catalysts developed in close cooperation with the Jagiellonian University in Cracow and the Jan Kochanowski University in Kielce. The catalysts have been designed with the effective treatment of tap water in mind, eliminating harmful chlorine compounds.
In 2008, in the village of Srebrna Gora in Lower Silesia, trichloroethylene was unexpectedly detected in the tap water. This is a carcinogenic compound conducive to the development of leukaemia and lymphoma, among others. The source of contamination turned out to be a factory which, 20 years earlier, had been used for the production of paints and solvents. In Poland alone, post-industrial pollution with chlorine compounds occurs several times a year. One method of preventing such a hazard—or rapidly eliminating its effects—may be the use of the new catalysts developed by the research groups. They are cheaper and more efficient than those used previously, and will enable a wider use of the hydrodechlorination reaction to effectively purify water, removing organic compounds containing chlorine.
Trichloroethylene, a colourless liquid, is a substance that is often used in industry as a solvent for fats. If it gets into a water tank, it does not dissolve, but sinks to the bottom, where it may remain for many years. If the tank is used as a drinking water intake over this period, at some point there may be a disturbance of the balance between the liquids, and the trichlorethylene deposits may be mixed with water taken up by the water supply. Another source of pollution by trichlorethylene is the atmosphere, as this compound evaporates and can be transmitted over long distances. In Europe, Chinese industry is among sources of danger.
"Trichloroethylene may enter our bodies when we consume food, while bathing, or even through inhaling," says Dr. Anna Srebowata (IPC PAS), and explains: "In small quantities, this compound causes abdominal pain and vomiting. However, if its concentration in the water exceeds the norm for a longer period, victims may suffer damage to the liver, heart and brain, as well as the development of cancer, particularly leukaemia, lymphoma and bladder cancer. Fast and cheap methods of removing trichlorethylene from water are therefore of great importance to society."
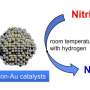
Ph.D. student Izabela I. Kami?ska (IPC PAS) explains the details of the research as follows: "Organic compounds of chlorine can be removed from water by a reaction known as hydrodechlorination. It uses small amounts of hydrogen and a suitably selected catalyst, usually palladium. The ensuing reaction causes organochlorine compounds in the water to be converted into non-toxic substances—saturated and unsaturated hydrocarbons. In the case of the reaction with trichlorethylene, these are ethane and ethylene."
Palladium is a very expensive element, so in palladium catalysts, what is important is the uniform distribution of nanoparticles of this metal on the surface of the support. This condition is met by the catalyst synthesized at the Jagiellonian University on a base of zeolite ZSM5—porous silica-alumina with an ordered structure. Rinsing out the silicon atoms and covering the material with palladium a catalyst is extremely effective in removing trichlorethylene from water, according to IPC PAS measurements. From a practical point of view, it is also important that in the new catalyst, palladium is immediately available in the reduced form, so it is ready for use immediately after synthesis.
"For many years, it was thought that zeolites were not suitable for the hydrodechlorination reaction because they are not resistant to the reaction mixture. In our experiments, we have shown that they can be used to create a highly efficient palladium catalyst. It is the high yield and speed of action that make the new catalyst so interesting, despite the use of palladium," says Dr. Srebowata.
An attractive alternative to precious metals are nickel catalysts. This material was obtained on a base of ordered carbon structures produced by chemists from the UJK. These structures are characterized by the presence of mesopores, i.e. pores with sizes of several dozen nanometres. At the IPC PAS, carbon materials prepared in this manner have had nickel applied to them, which has then been reduced to the metallic phase.
"We have demonstrated that a catalyst composed of nickel supported on mesoporous activated carbon removes trichlorethylene from water. Although the process of hydrodechlorination takes place slightly more slowly than in the case of palladium catalysts, there are important economic factors, because nickel is a relatively inexpensive element. In addition, our new catalyst works even in small amounts, and degrades very slowly, which confirms our belief that it can be an excellent replacement for expensive noble metals," notes Dr. Srebowata.
More information: Anna Śrębowata et al. Catalytic removal of trichloroethylene from water over palladium loaded microporous and hierarchical zeolites, Applied Catalysis B: Environmental (2016). DOI: 10.1016/j.apcatb.2015.08.025
Journal information: Applied Catalysis B: Environmental
Provided by Polish Academy of Sciences