October 6, 2015 report
Covalent organic framework that has highest reported stability and can be modified for organocatalysis
(Phys.org)—A group of researchers from the Institute for Molecular Science, National Institutes of Natural Sciences in Japan report a novel covalent organic framework (COF) that is crystalline, highly stable in water, strong acids, and strong bases, and has a remarkably high 2D surface area. This COF was modified with catalytic subunits and used as a catalyst under inert conditions for Michael addition reactions using a variety of reactants. Their work is published in Nature Chemistry.
Covalent organic frameworks are crystalline organic structures that are porous. They are an extended organic framework similar to graphene. COFs can serve as an organic platform for other reactions or, in the case of this research, as a catalyst. This catalyst was metal-free, and when modified with chiral catalytic arms, produced entioselective products. Furthermore, this catalyst allowed for a Michael reaction under relatively mild conditions and was able to be recycled multiple times.
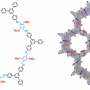
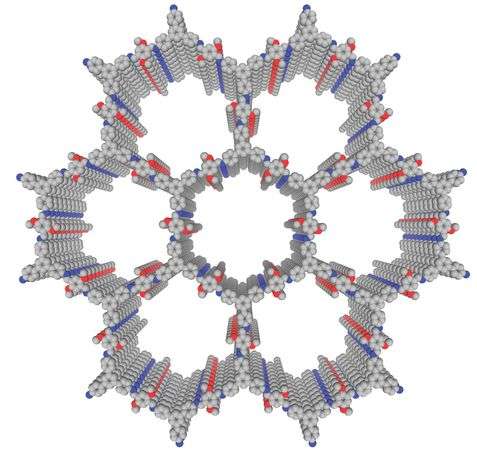
The ideal COF has stability, crystallinity, and porosity. Prior research on COFs shows that it is difficult to optimize all three of these properties in one covalent framework. Importantly, crystallinity and porosity in 2D frameworks depend on interlayer interactions. Hong Xu, Jia Gao and Donglin Jiang designed a COF that has additional delocalization over the phenyl groups in an effort to enhance interlayer interactions via pi-stacking. Their COF is comprised of triphenylbenzene (TAPB) and dimethoxyterephthaldehyde (DMTA) subunits. When combined, the aldehydes on DMTA react with the amines on TAPB to form an imine which is conjugated with the dimethoxy phenyl groups in DMTA. This added interlayer stability promotes crystallization.
The new TAPB-DMTA COF's stability was tested using thermogravimetric analysis after samples were subjected to various solvents, including water, strong acid, and strong base, for one week. Results showed that there was no loss in weight in the COF in water or the organic solvents tested (DMF, DMSO, THF, MeOH, and cyclohexanone). Twelve molar HCl resulted in 85 wt% of the original COF, and 14M NaOH resulted in 92 wt%. The COF was still 72% of its original weight after being subjected to boiling water for a week. Additionally, powder x-ray diffraction and surface area measurements showed that the COF maintained its original crystal structure and only decreased somewhat in surface area. IR studies showed that the bonds remained intact.
Additionally, crystallinity and porosity were investigated using powder x-ray diffraction analysis. Analyses and simulation studies confirmed the crystalline structure. A nitrogen-sorption profile indicated that the new COF was likely a mesoporous material. Additionally, Xu, et al. confirmed that the COF had a very high 2D surface area. Taken together, this new COF has remarkable stability and high crystallinity and porosity. With such excellent features, this COF should make a good platform for catalysis.
To make the TABP-DMTA COF into an entioselective catalyst, Xu, et al. functionalized the COF walls with chiral centers on organocatalytic sites. These chiral organocatalytic sites [(S)-Py] were attached to some of the methoxy groups on the DMTA subunits. Not all methoxy groups were substituted, and, indeed, the number of groups that were substituted with (S)-Py affected the catalytic activity. Characterization confirmed the structure of the catalytic COF and that the overall structure of the COF was maintained after functionalization. The organocatalytic sites were incorporated into the walls of the COF and, based on number of sites within a pore, they create nanopores of varying sizes.
To test the organocatalyst, Xu, et al. looked at Michael addition reactions using various reactants. While Michael addition reactions are typically conducted in organic solvents, in this case, water was used as it has more practical utility. The reaction was conducted in water at 25oC at near atmospheric pressure. Because the catalytic COF was insoluble, this allowed for recovery and reuse of the catalyst. The model Michael reaction involved reacting cyclohexanone and β-nitrostyrene. This resulted in 100% conversion, 92% enantiomeric excess, and 90/10 diastereoselectivity.
The authors note that this is the first example of a heterogenous catalyst based on a crystalline porous material that has been used for the activation of low-activity ketones in a Michael addition reaction.
They investigated how their catalytic COF worked with Michael addition reactions using different reactants. All had good conversion and selectivity. Four-methoxy-β-nitrostyrene had the best selectivity with 96% e.e. and 94/6 diastereoselectivity after reacting for 26 hours. Kinetic studies indicated first-order with respect to the nitrostyrene reactant. Additionally, the catalyst retained its reactivity after five cycles, although reaction time increased with each cycle.
"One of the key issues of COFs is the combination of stability, crystallinity and porosity," says principle investigator Donglin Jiang, "This study would open a platform for functional explorations and applications of COFs."
More information: "Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts", Hong Xu, Jia Gao and Donglin Jiang, Nature Chemistry, DOI: 10.1038/NCHEM2352
Abstract
The periodic layers and ordered nanochannels of covalent organic frameworks (COFs) make these materials viable open catalytic nanoreactors, but their low stability has precluded their practical implementation. Here we report the synthesis of a crystalline porous COF that is stable against water, strong acids and strong bases, and we demonstrate its utility as a material platform for structural design and functional development. We endowed a crystalline and porous imine-based COF with stability by incorporating methoxy groups into its pore walls to reinforce interlayer interactions. We subsequently converted the resulting achiral material into two distinct chiral organocatalysts, with the high crystallinity and porosity retained, by appending chiral centres and catalytically active sites on its channel walls. The COFs thus prepared combine catalytic activity, enantioselectivity and recyclability, which are attractive in heterogeneous organocatalysis, and were shown to promote asymmetric C–C bond formation in water under ambient conditions.
Journal information: Nature Chemistry
© 2015 Phys.org