Researcher discusses capturing carbon in the presence of water with MOFs and COFs
Burning through a tank of gasoline in a typical automobile releases about 81 kilograms of carbon dioxide into the atmosphere. This adds up to a staggering 10 billion tons of carbon dioxide emission each year from cars alone. Capturing and either sequestering these carbon emissions or converting them into valuable chemical products is one way to combat the effects of global climate change. Highly promising candidates for capturing and containing carbon dioxide and other gases are crystalline extended systems with sponge-like storage capabilities. Two major classes of these systems are metal organic frameworks (MOFs) and covalent organic frameworks (COFs) but a major challenge in carbon dioxide capture has been to carry it out in the presence of water; that challenge is now being met by MOFs and COFs.
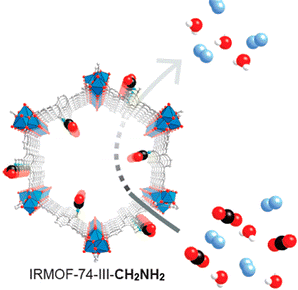
Speaking at the Fall 2015 meeting of the American Chemical Society in Boston, Berkeley Lab and University of California at Berkeley chemist Omar Yaghi, the inventor of MOFs, described the use of another technology he pioneered, "reticular chemistry," to produce a series of compounds called "IRMOF-74-III," which are effective for selective carbon dioxide capture in the presence of water. He also described the design of MOFs and COFs based on porphyrins that can be fine-tuned for efficient electrochemical carbon dioxide reduction. The "IR" in IRMOF stands for isoreticular, which essentially means that the molecular system is "stitched together" into a netlike structure through strong chemical bonds.
"Our IRMOF-74-III compounds feature different groups in the pore interior, including methyl, aromatic, primary and secondary amines, that can be covalently functionalized with primary amines and used for the selective capture of carbon dioxide in 65-percent relative humidity," said Yaghi, who holds joint appointments with Berkeley Lab's Materials Sciences Division and UC Berkeley's chemistry department. He is also co-director of the Kavli Energy NanoScience Institute (Kavli-ENSI).
Breakthrough experiments showed that IRMOF-74-III compounds are especially efficient at removing carbon dioxide from wet nitrogen gas streams in record efficiency and time while fully preserving the IRMOF structure.
Ultimately, the captured carbon dioxide should be converted to fuels and valuable feedstock chemicals, Yaghi said.
"Our MOF conversion technique allows for nanometer precision of catalyst-loading to optimize our systems for high activity. The ability to covalently link catalysts as an integral part of our COF/MOF structures to design and optimization, and modulation of the electronic structure of the catalyst to give very high performance."
Provided by Lawrence Berkeley National Laboratory