Flexibility and self-repair of the cell skeleton
Researchers at the CEA, the CNRS and the Joseph Fourier University in Grenoble have discovered several mechanical properties, as fascinating as they are unexpected, in microtubules, the main elements in the cell skeleton, and especially their capability of adapting to stress and of self-repair. These discoveries have been possible thanks to the creation of a microfluidic device that makes it possible to attach, fold and measure distortions in microtubules. Microtubules play a crucial role in various processes such as cell division and neuron activity. Their repair dynamic could serve as an inspiration for materials engineering. These results were published in Nature Materials magazine on 7 September 2015.
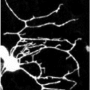
Microtubules, the main constituents of internal cell architecture, possess a rigidity that is one hundred times greater than that of other constituents of the cytoskeleton. For this reason, they travel through intracellular space in a virtually straight line, serving as the route for transporting proteins from the centre of the cell to its periphery. The regulating mechanisms of their mechanical properties are still virtually unknown, however. Their rigidity can be explained by their structure, that of a hollow tube, an efficient way, well-known to bicycle manufacturers, of constructing rigid elements using the least possible amount of material. These mechanical properties could not be studied in detail hitherto since the appropriate tools were lacking. A microfluidic device that can attach itself to microtubules and bend them has been perfected by researchers at the Plant Cell Physiology Laboratory (CNRS/CEA/INRA/Joseph Fourier University) and the Interdisciplinary Physics Laboratory (CNRS/Joseph Fourier University).
Scientists isolated the microtubules in cells in order to overcome the complexity of the intracellular environment and were thus able to study their intrinsic mechanical properties under simple conditions. They then used very weak hydrodynamic flows to apply slight, and well-controlled pressure so as to bend them gently. This is when they discovered that as the pressure cycles were applied repeatedly, the microtubules bent to an increasingly great extent but did not break. The application of external pressure makes them increasingly flexible. Their structure thus seems to be capable of reorganising itself and adapting to pressure. Even more surprising, microtubules are capable of rediscovering their initial rigidity if pressure is interrupted for a few minutes. They repair themselves spontaneously.
The structure of microtubules consists of 13 filaments that adhere to each other to form a hollow tube. These filaments are themselves composed of molecules of self-assembled tubulins. In order to better understand the adaptation and repair mechanisms of microtubules, researchers used tubulins in various concentrations and of different colours. They were thus able to determine the fact that the quasi-crystalline structure of microtubules can contain defects and that these constitute weaknesses. It is from these weak points that filaments break apart under stress, rendering the microtubules more flexible. In the opposite case, during rest phases, the filaments can incorporate new tubulin molecules and thus repair a damaged structure, enabling it to recover its original rigidity.
This innovative research is the first stage of a better understanding of how microtubules function. Microtubules lie at the heart of the regulation of numerous cell processes such as cell division or neuron activity. Far from the classical view according to which microtubules only link together at their tips, it would appear that the self-assembly mechanisms of the filaments of which they consist offer a whole range of mechanical and biochemical properties that were unsuspected hitherto and whose contribution to the multiple functions of microtubules still remains to be elucidated. Furthermore, the materials of which living cells consist have become a source of inspiration for engineering. Microtubules demonstrate the unique properties of self-repair and mechanical adaptation that are specific to their status as dynamic polymers. They could serve as the basis for the design of new devices for applications as varied as the textile or electronics industries of the future.
Journal information: Nature Materials
Provided by CEA