New chemistry makes strong bonds weak
Researchers at Princeton have developed a new chemical reaction that breaks the strongest bond in a molecule instead of the weakest, completely reversing the norm for reactions in which bonds are evenly split to form reactive intermediates.
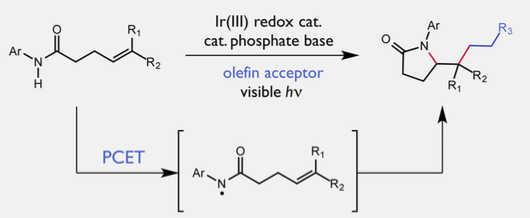
Published on July 13 in the Journal of the American Chemical Society, the non-conventional reaction is a proof of concept that will allow chemists to access compounds that are normally off-limits to this pathway. The team used a two-component catalyst system that works in tandem to selectively activate the strongest bond in the molecule, a nitrogen-hydrogen (N-H) bond through a process known as proton-coupled electron transfer (PCET).
"This PCET chemistry was really interesting to us. In particular, the idea that you can use catalysts to modulate an intrinsic property of a molecule allows you to access chemical space that you couldn't otherwise," said Robert Knowles, an assistant professor of chemistry who led the research.
Using PCET as a way to break strong bonds is seen in many essential biological systems, including photosynthesis and respiration, he said. Though this phenomenon is known in biological and inorganic chemistry settings, it hasn't been widely applied to making new molecules—something Knowles hopes to change.
Given the unexplored state of PCET catalysis, Knowles decided to turn to theory instead of the trial and error approach usually taken by synthetic chemists in the initial stages of reaction development. Using a simple mathematical formula, the researchers calculated, for any pair of catalysts, the pair's combined "effective bond strength," which is the strength of the strongest bond they could break. Because both molecules independently contribute to this value, the research team had a high degree of flexibility in designing the catalyst system.
When they tested the catalyst pairs in the lab, the researchers observed a striking correlation between the "effective bond strength" and the reaction efficiency. While effective bond strengths that were lower or higher than the target N-H bond strength gave low reaction yields, the researchers found that matching the strengths promoted the reaction in very high yield.
"To see this formula actually working was really inspiring," said Gilbert Choi, a graduate student in the Knowles lab and lead author on the work. Once he identified a successful catalyst system, he explored the scope of the reaction and its mechanism.
The researchers think that the reaction starts with one of the catalysts, a compound called dibutylphosphate, tugging on a hydrogen atom, which lengthens and weakens the N-H bond. At the same time, the other catalyst, known as a light-activated iridium complex, targets the weakened bond and plucks off one electron from the two-electron bond, slicing it down the middle.
Once the bond is split, the reactive nitrogen intermediate goes on to form a new carbon-nitrogen bond, giving rise to structurally complex products. This finding builds on work the Knowles lab published earlier this year also in the Journal of the American Chemical Society on a similar reaction that used a more sensitive catalyst system.
Their research has laid a solid foundation for PCET catalysis as a platform for developing new reactions. "My sincere view is that ideas are a lot more valuable than reactions," Knowles said. "I'm optimistic that people can use these ideas and do things that we hadn't even considered."
More information: Choi, G. J.; Knowles, R. R. "Catalytic Alkene Carboamination Enabled by Oxidative Proton-Coupled Electron Transfer." 2015, J. Am. Chem. Soc., Article ASAP.
Journal information: Journal of the American Chemical Society
Provided by Princeton University