Novel battery uses light to produce power
To move the world toward sustainability, scientists are continuing to explore and improve ways to tap the vast power of sunlight to make fuels and generate electricity. Now they have come up with a brand-new way to use light—solar or artificial—to drive battery power safely. Their "photo battery," reported in ACS' The Journal of Physical Chemistry C, uses light and titanium nitride for the anode.
Metal-ion batteries such as those based on lithium ions run most of our gadgets. But they take a long time to charge. They can also overheat and catch fire if they're defective or damaged. These problems are often related to the unstable material used for the anode, the negative side of the battery. Musthafa Ottakam Thotiyl and colleagues wanted to address these flaws in a unique way.
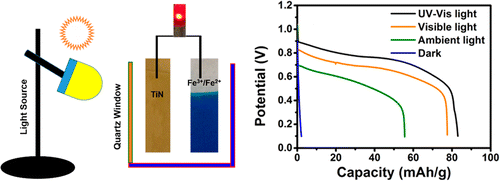
The researchers developed a battery with a titanium nitride photoanode that is highly stable and thus far safer than conventional options. Under normal indoor lighting, it discharged electric current and recharged within 30 seconds without an external power source. The photo battery worked for more than 100 cycles and could power a light-emitting diode (known as an LED). Although not yet strong enough to run commercially available devices, the researchers say their design is a promising first step toward a more sustainable and safer battery technology.
More information: Chemically Chargeable Photo Battery, J. Phys. Chem. C, Article ASAP. DOI: 10.1021/acs.jpcc.5b02871
Abstract
Here we show a surrogate strategy for power production, wherein light is used to actuate a discharge chemistry in the cathode of an aqueous rechargeable battery (ARB). The proposed photo battery consists of a titanium nitride photoanode, promising cathode material iron(III) hexacyanoferrate(II) as the battery active species and Na2S2O8 as the chemical charging agent. The photo battery delivered negligible capacity in the dark and the capacity shot up to 77.8 mAh/g when artificially shined light, confirming that the battery chemistry is light driven. In the ambient light, the device retained 72% of its artificial light discharge capacity with a stable cycling for more than 100 cycles. Further, an unprecedented means for charging the battery rapidly is presented using Na2S2O8 and it revitalized the battery in 30 s without any external bias. This methodology of expending a photoanode extends to a battery that is free from dissolution of active materials, irreversible structural changes, spontaneous deinsertion reactions, and safety concerns commonly encountered in the state of the art anode materials in ARBs. Apart from bringing out a sustainable way for power production, this device opens up avenues for charging the battery in the likely events of electrical input unavailability, while solving the critcial issues of longer charging time and higher charging voltage.
Journal information: Journal of Physical Chemistry C
Provided by American Chemical Society