Team first to model atomic structures of three bacterial nanomachines
Researchers at UCLA's California NanoSystems Institute have become the first to produce images of the atomic structures of three specific biological nanomachines, each derived from a different potentially deadly bacterium—an achievement they hope will lead to antibiotics targeted toward specific pathogens.
The scientists used a leading-edge technology called cryo electron microscopy, or cryoEM, to reveal the form and function of these important structures. Papers on their findings were published in three top-tier journals: Nature, Cell, and Nature Structural and Molecular Biology.
Two of the nanomachines are structures called contractile ejection systems, which their bacteria use to transfer toxic molecules into healthy cells to usurp them for their own purposes, to attack rival bacteria by delivering toxins into them, and other functions. These structures have sheath–tube assemblies that create openings in the outer membranes of target cells through which they can insert toxic molecules.
The third nanomachine—different from the other two—is a pore structure that delivers deadly anthrax toxin into mammalian cells, once the anthrax bacteria is in the bloodstream. This mechanism is how anthrax bacteria activate the disease in an infected animal or person.
How the nanomachines work had been poorly understood, but the UCLA researchers used a cryoEM equipped with a special camera called a direct electron detector to produce highly detailed images. The scientists hope the new information about how they function will enable them to create antibiotics that target bacterial pathogens.
The team, led by Hong Zhou, professor of microbiology, immunology and molecular genetics, and of chemistry and biochemistry, runs the Electron Imaging Center for Nanomachines laboratory, which is based at CNSI and houses UCLA's Titan Krios electron microscope—a highly sophisticated and rare cryoEM.
"As the centerpiece of our electron microscopy core lab, the cryo electron microscope is enabling exploration of new territory in molecular biology," said Jeff Miller, director of the California NanoSystems Institute. "These unprecedented images enable us to understand the actual workings of these remarkable structures."
Anthrax toxin
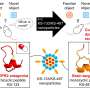
In a paper published online by Nature, Professor Zhou and his team reported that they were the first to determine the atomic structure of the anthrax toxin pore, the major disease molecule of Bacillus anthracis, the bacterium that causes the disease anthrax in humans and animals. The anthrax toxin pore's atomic structure is mushroom-shaped with a gate inside the "shaft."
The finding confirms how the disease affects cells. When healthy cells encounter nanoscale objects in the body, they assume the objects are nutrients and absorb them. Like a Trojan horse, the toxin pore appears to the cells as something beneficial—in this case, a nutrient—and is taken in by the cell. But once inside the cell, the pore senses the change to a more acidic environment, which opens the pore's gate and releases the anthrax toxin molecule into the cell.
"This is a very important step toward understanding this mechanism, and it is essential for any anthrax countermeasure," Zhou said. "It also informs our understanding of the mechanisms of other toxins that function like anthrax, which could lead to other targeted antibiotic drugs."
Tularemia type VI secretion system
Another nanomachine was described by Dr. Marcus Horwitz, a UCLA professor of medicine and of microbiology, immunology and molecular genetics, who worked with Zhou's team. In a study published in the journal Cell, the scientists reported the first atomic resolution model of any type VI secretion system, or T6SS, a nanomachine found in roughly 25 percent of gram-negative bacteria.
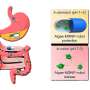
Gram-negative bacteria are responsible for diseases such as cholera, salmonellosis, Legionnaires' disease and melioidosis, and severe infections including gastroenteritis, pneumonia and meningitis. For the new study, the scientists examined Francisella tularensis, a bacterium that causes tularemia and is of great concern as a potential bioterrorism agent.
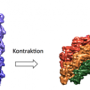
Built from component proteins, the T6SS nanomachine has an atomic structure that resembles a piston. When F. tularensis is taken up into a type of white blood cell called a macrophage it is surrounded by a bubble-like membrane, a structure known as a phagosome. The T6SS nanomachine then assembles inside the bacterium, where it plunges a tube through the bacterial wall and the membrane of the phagosome into the cytoplasm, the substance inside the macrophage. This enables the bacterium to escape the phagosome into the cytoplasm, where it can complete its lifecycle and multiply. Soon, the macrophage fills with bacteria and ruptures, freeing the bacteria to infect other cells. Thus, the T6SS is a novel target for antibiotics against this bacterium, and against others that use it to survive within host cells or to combat rival bacteria.
"We are already identifying drug molecules that target the F. tularensis T6SS," Horwitz said. "Knowing how this structure works guides us in selecting drug molecules that block its assembly or function. The overall goal is to find new antibiotics that directly target this top-tier bioterrorism agent and other gram-negative bacteria with a T6SS such as Vibrio cholerae, Pseudomonas aeruginosa, Burkholderia pseudomallei, and pathogenic Escherichia coli."
Horwitz and his team could potentially also develop wider-spectrum drugs that work on many different gram-negative pathogens that have in common a T6SS.
Pseudomonas aeruginosa
In humans and animals, a bacterium called Pseudomonas aeruginosa causes infectious diseases that lead to generalized inflammation and sepsis, a dangerous infection of the blood. A team led by Zhou and Miller discovered the atomic structures of R-type pyocins, contractile ejection systems of Pseudomonas aeruginosa. Their findings were published online by Nature Structural and Molecular Biology.
R-type pyocins are used by the bacterium to rapidly insert their nanotubes, like battering rams, into the cell membranes of competing bacteria to kill the competitors, giving Pseudomonas aeruginosa easier access to nutrients. These pyocins appear to create a channel in the outer envelope of the target bacteria, which essentially acts to weaken and kill it. This ability has made R-type pyocins the focus of research into possible antimicrobial and bioengineering applications, and scientists believe they could be engineered to give drugs a powerful antibacterial component.
"The R2 pyocin is an extraordinary molecular machine that uses energy from its own biological battery to function," said Miller, who also is a professor of microbiology, immunology and molecular genetics. "It is ideal for engineering targeted antibiotics that kill the bad bacteria without disrupting a patient's protective gut bacteria."
The scarcity of the technology and the expertise needed to use it make CNSI one of the world's few facilities capable of imaging atomic structures like these nanomachines at atomic-level resolution, which is why researchers from around the world come to UCLA to use the Electron Imaging Center for Nanomachines, a fee-for-service laboratory open to any scientist in academia or industry.
Other UCLA researchers who contributed to the three papers were Daniel Clemens, adjunct professor of medicine; Xuekui Yu, adjunct assistant professor of microbiology, immunology and molecular genetics; Peng Ge, a research associate; Bai-Yu Lee, an associate researcher; and Jiansen Jiang, a postdoctoral fellow. Bradley Pentelute of Massachusetts Institute of Technology, R. John Collier of Harvard University Medical School, Dean Scholl of AvidBiotics and Petr Leiman of the Ecole Polytechnique Federale de Lausanne's Institute of Physics of Biological Systems were the other co-authors.
Journal information: Cell , Nature , Nature Structural and Molecular Biology
Provided by University of California, Los Angeles