Soy: It's good for eating, baking—and cleaning up crude oil spills
If you've studied ingredient labels on food packaging, you've probably noticed that soy lecithin is in a lot of products, ranging from buttery spreads to chocolate cake. Scientists have now found a potential new role for this all-purpose substance: dispersing crude oil spills. Their study, which could lead to a less toxic way to clean up these environmental messes, appears in ACS Sustainable Chemistry & Engineering.
Ram B. Gupta and colleagues explain that applying chemical dispersants is one of the most effective ways to help get rid of oil spills quickly. The dispersants work by breaking down oil into small droplets that bacteria can then degrade. But some dispersants can be harmful to cleanup crews and aquatic life. So Gupta's team turned to a natural, more benign surfactant—an agent that hastens the absorption of liquids by forcing them to form small droplets—to see if it could do the same job without the negative health effects.
The researchers separated crude soy lecithin, a very effective surfactant used in foods, into its lipid components. Then, they tested how well these lipids could break down oil in water in the lab. They found the compounds worked as well as or slightly better than two commercial dispersants.
More information: Soybean Lecithin as a Dispersant for Crude Oil Spills, ACS Sustainable Chem. Eng., Article ASAP. DOI: 10.1021/acssuschemeng.5b00027
Abstract
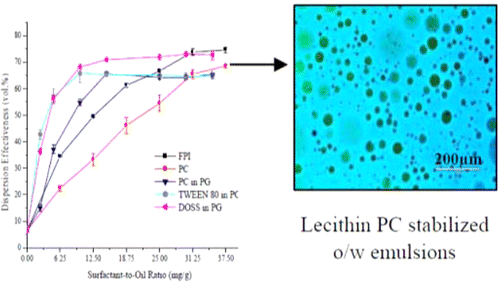
The toxicity of oil spill dispersants to marine organisms has necessitated the search for alternative dispersant formulations that are environmentally benign. Soybean lecithin, a well-known surface active agent in the food industry, is effective at stabilizing oil-in-water emulsions. In addition to its excellent emulsification properties, it is biodegradable, less toxic than the traditional chemical dispersants, and ecologically acceptable. In this study, soybean lecithin was used to formulate dispersants for crude oil spill application. Soybean lecithin was fractionated into phosphatidylinositol (PI) and phosphatidylcholine (PC) enriched fractions using ethanol. The fractionated PI was deoiled and characterized with Fourier transform infrared spectroscopy (FT-IR). The crude soybean lecithin (CL) and the fractionated PI and PC were solubilized in water and their dispersion effectiveness determined using the U.S. EPA's baffled flask test. The dispersion effectiveness of these solubilized dispersants was compared with that of solid crude lecithin (SL). The dispersion effectiveness of PC was found to be higher than those of SL, CL, and PI at all the surfactant-to-oil ratios (SORs) tested. However, when the fractionated PI was modified or "functionalized" (FPI) with additional hydroxyl groups to alter the hydrophilic–lipophilic balance (HLB), its dispersion effectiveness improved remarkably and was higher than that of PC. At higher SORs (>28 mg/g), the dispersion effectiveness of FPI was slightly higher than that of solubilized DOSS and Tween 80 in propylene glycol. The dispersion effectiveness of PC and FPI on Texas (TC) and light crude (LC) oil samples were almost the same. PC and FPI performed better at the higher salinity of 3.5 wt % than the lower salinities of 0.8 and 1.5 wt %. The findings from this study suggest that dispersants formulated from fractionated PI and PC have the potential to replace traditional dispersant formulations.
Provided by American Chemical Society

















