March 9, 2015 weblog
Researchers explore fading red in Van Gogh art

While debate over whether a dress was blue and black or white and gold has caused an Internet storm of talk about color, discussions in scientific circles raise questions about red, asking why are Van Gogh's reds turning white? Researchers have answers. Scientists in Belgium believe they can explain why these reds are turning white, and have explored the degradation process that causes the color bleaching. The University of Antwerp team looked at a microscopic sample of Van Gogh's "Wheat Stack Under a Cloudy Sky."
Red lead or r minium (Pb3O4), thought to be one of the earliest synthetically produced pigments in antiquity, was used extensively by Van Gogh in his paintings, said Chemistry World. "Red lead is most familiar to us in orange-red rustproof paint. Artists have treasured the brilliant color of this pigment for their paintings since ancient times," said the University of Antwerp site report about their research. Their discovery sheds new light on the bleaching process of red lead.
Matthew Gunther in Chemistry World said the "researchers think that when red lead is exposed to light it is converted into plumbonacrite, which reacts with carbon dioxide to form hydrocerussite and cerussite."
According to the University of Antwerp research group, Antwerp X-ray Analysis, Electrochemistry and Speciation, reporting on the investigation, minium is a lead oxide whose composition is Pb3O4 and whose color varies over time. Sometimes the color darkens or blackens as the red lead pigment is converted to plattnerite (β-lead dioxide) or galena (lead sulfide). At other times, the color will lighten or bleach due to the conversion of red lead to lead sulfate or lead carbonate.
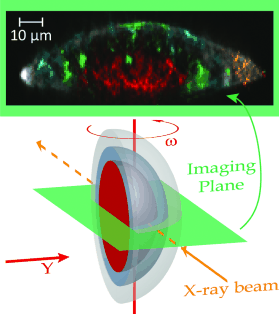
Koen Janssens headed the University of Antwerp team; they were able to clarify the degradation process of red lead that causes this color bleaching. 'Wheat Stack under a Cloudy Sky'1889, is oil on canvas, from the Kröller Müller Museum, Netherlands; the team examined a microscopically small sample of the painting. They used X-ray powder diffraction mapping and tomography techniques. Doing so enabled them to determine the distribution of different crystalline compounds. In contrast to conventional X-ray crystallographic methods, they said, their methods gave them a depth profile of the sample's composition without cutting it open.
That is how they found an unexpected compound, the rare lead carbonate mineral, plumbonacrite (3•PbCO3•Pb(OH)2•PbO). "This is the first time that this substance has been found in a painting from before the mid-twentieth century," said Frederik Vanmeert, first author of the paper. Their paper appeared in Angewandte Chemie, published by the German Chemical Society. The title of their work is "Plumbonacrite Identified by X-ray Powder Diffraction Tomography as a Missing Link during Degradation of Red Lead in a Van Gogh Painting." The article was published online Feb. 20.
Why does this research matter? Allison Meier, who writes about visual art, discussed this in the art forum Hyperallergic. She said, "continued research on the chemistry of van Gogh's pigments could have a wider influence on art conservation. And importantly, it could influence the way his paintings are displayed in light, knowing that the rare mineral in the red may fade from the colors the artist originally envisioned."
Gunther quoted Philippe Walter, director of the Laboratory of Molecular and Structural Archaeology in France: "For the conservation of works of art, it is important to look at this phenomenon to be sure to take care about the quantity of light [in museums]."
More information: Plumbonacrite Identified by X-ray Powder Diffraction Tomography as a Missing Link during Degradation of Red Lead in a Van Gogh Painting, Angewandte Chemie, onlinelibrary.wiley.com/doi/10 … e.201411691/abstract
Abstract
Red lead, a semiconductor pigment used by artists since antiquity, is known to undergo several discoloration phenomena. These transformations are either described as darkening of the pigment caused by the formation of either plattnerite (β-PbO2) or galena (PbS) or as whitening by which red lead is converted into anglesite (PbSO4) or (hydro)cerussite (2 PbCO3⋅Pb(OH)2; PbCO3). X-ray powder diffraction tomography, a powerful analytical method that allows visualization of the internal distribution of different crystalline compounds in complex samples, was used to investigate a microscopic paint sample from a Van Gogh painting. A very rare lead mineral, plumbonacrite (3 PbCO3⋅ Pb(OH)2⋅PbO), was revealed to be present. This is the first reported occurrence of this compound in a painting dating from before the mid 20th century. It constitutes the missing link between on the one hand the photoinduced reduction of red lead and on the other hand (hydro)cerussite, and thus sheds new light on the whitening of red lead.
Journal information: Angewandte Chemie
© 2015 Phys.org