Researchers clarify structure and function of new enzyme that reduces sulfite even faster
Sulfites are sulfurous substances that occur naturally. They are poisonous for many life forms even at small concentrations. Sulfites and sulfur dioxide are also added to wine and dried fruit as preservatives that inhibit the growth of unwanted microorganisms, increasing the shelf-life of these products. The biochemists Prof. Dr. Oliver Einsle and Dr. Bianca Hermann from the University of Freiburg have teamed up with researchers from the Technische Universität Darmstadt for a project in which they characterised a bacterial enzyme that reduces sulfite up to one hundred times faster than any other known enzyme.
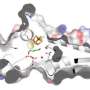
The researchers were able to clarify the high-resolution crystal structure of the enzyme complex MccA and the molecular details of its reaction mechanism. Their findings explain why MccA is able to convert sulfite so quickly, meaning that this enzyme could be used more frequently in biotechnology in the future. Customised microorganisms capable of high-speed sulfite reduction could be employed for desulfurization under mild conditions, for example.
The results of the scientists' research have now been published in Nature.
Some bacteria use sulfite for their metabolism - for example, for respiration when there is no oxygen. Bacteria harvest energy by reducing sulfite to sulfide, during which they split off all oxygen atoms as water. One of the bacteria that use sulfur for respiration is Wolinella succinogenes. This organism can be found in the rumen of ruminants, where it produces MccA. MccA is a metalloprotein that contains 24 haem groups with iron ions. It also consists of three subunits, making it a trimer.
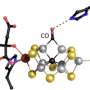
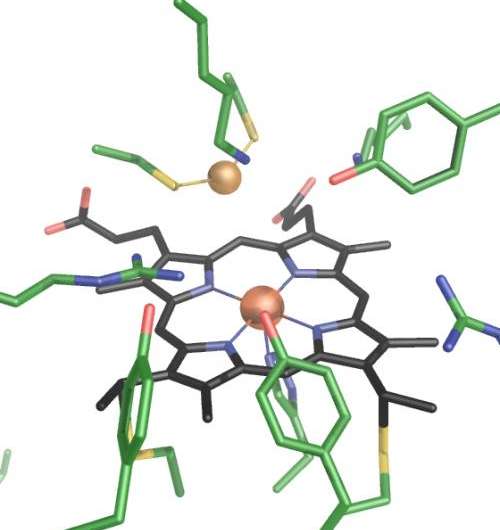
The scientists were able to decode the enzyme's structure, revealing a previously unknown active centre for sulfite reduction. This centre consists of one of the haem groups combined with a copper ion, which is bound to two residues of cysteine, a sulfurous amino acid. Through its position, the copper ion prevents sulfite ions from binding to the enzyme. Instead, sulfur dioxide binds to the active centre because it formally contains one less water molecule and thus requires less space, so to say. This initial step is what distinguishes the reduction mechanism of MccA from other enzymes that break down sulfite. MccA then reduces sulfur dioxide to sulfide through further dehydration. The scientists were able to detect sulfur dioxide and sulfur monoxide in the enzyme's structural model, from which they concluded this new mechanism.
More information: Bianca Hermann, Melanie Kern, Luigi La Pietra, Jörg Simon & Oliver Einsle. 2015. "The octahaem MccA is a haem c-copper sulfite reductase." Nature. DOI: 10.1038/nature14109
Journal information: Nature
Provided by Albert Ludwigs University of Freiburg