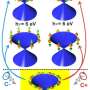
Losing 1 electron switches magnetism on in dichromium
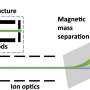
The scientists used the unique Nanocluster Trap experimental station at the BESSY II synchrotron radiation source at Helmholtz-Zentrum Berlin and published their results in the Journal Angewandte Chemie.
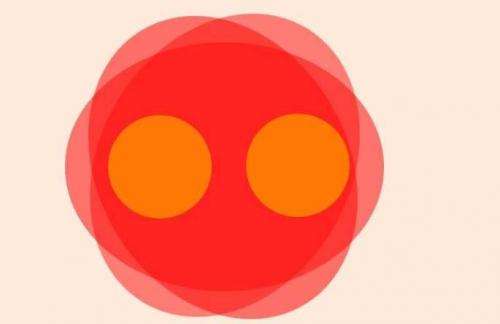
The electronic structure and bonding of seemingly simple diatomic molecules like dichromium has puzzled scientist for decades. In surprisingly many cases, the ground state of these smallest molecules is still unknown even after a century of quantum mechanics. Because of the enormous computational challenge associated with the correct description of low-lying excited states and multiple bonds, the sextuple bond in the low-spin ground state of neutral Cr2 molecules has become a benchmark criterion in electronic structure calculations. In a joint effort, an international team of scientists from Berlin, Freiburg and Fukuoka has now provided the first direct experimental proof of an unexpected high spin ground state of Cr2+, the cationic cousin of Cr2.
Dramatic effect on magnetism
The team studied the effect of x-ray magnetic circular dichroism on free Cr2+ ions that were stored at 18 K in a dedicated cryogenic ion trap. This effect gives direct experimental insight into spin coupling and localization of the relevant valence electrons. For their experiments, the scientists used the unique Nanocluster Trap experimental station that is available at beamline UE52-PGM of the BESSY II synchrotron radiation source at Helmholtz-Zentrum Berlin.
Localisation of ten valence electrons
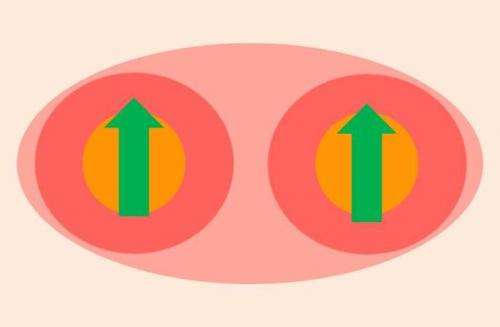
Even though only one out of twelve electrons is removed when ionizing Cr2 the molecule reacts dramatically, with complete localization of all ten 3d electrons and with maximum spin coupling. This turns an archetypal antiferromagnet ferromagnetic. "It's a dramatic effect we see," says team leader Tobias Lau. "Its particular spin configuration can be interpreted as a result of indirect exchange coupling, where the two groups of localized electrons "talk" to each other via a single bonding electron as a messenger that controls the parallel alignment of all their spins," says Vicente Zamudio-Bayer who conducted this work as part of his PhD thesis at HZB and TU Berlin and who now continues his research as a postdoc in the Freiburg group.
Almost the same bonding energy
While in the neutral molecule all twelve valence electrons participate in bonding and create a short, unusual sextuple bond, the cation is only bound by one single electron with an almost doubled bond distance but almost the same bond energy. These significantly different bonding situations illustrate the fragile and untypically weak multiple bond in dichromium. They can be visualized as a change from a short and tight multiple bond to which all valence electrons contribute, to a long and loose single bond with all electrons except one localized at both ends. Combining their new results with earlier findings, the scientists can now even give relative energies of the excited states that have caused much confusion in the correct description of this molecular ion, a fact that will facilitate future theoretical approaches.
Cooperation and experimental set up
The experimental setup that was used for this research is operated jointly by Helmholtz-Zentrum Berlin, Universität Freiburg, and Kyushu University in Fukuoka, Japan. It is currently the only setup worldwide that provides the opportunity to investigate with x-ray spectroscopy ultralow density samples of a broad range of gaseous and size-selected molecular ions, clusters and complexes trapped at cryogenic temperatures in a strong magnetic field. This unique setup at BESSY II is currently upgraded in a BMBF-funded project of Universität Freiburg for even lower temperature and increased sensitivity, with the promise for more of these exciting results to come.
More information: Angewandte Chemie. DOI: 10.1002/anie.201411018
Journal information: Angewandte Chemie
Provided by Helmholtz Association of German Research Centres