New catalyst to create chemical building blocks from biomass
University of Tokyo researchers have developed a novel selective catalyst that allows the creation of several basic chemicals from biomass instead of petroleum. This discovery may lead to the use of plant biomass as a basic feedstock for the chemical industry.
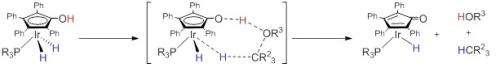
The new catalyst developed by Professor Kyoko Nozaki's research group at the Graduate School of Engineering enables selective cleaving (hydrogenolysis) of carbon-oxygen (C-O) single bonds in phenols and aryl methyl ethers, two of the main components of lignin. Lignin is a major component of plant dry matter and has the potential to replace petroleum as the primary source of basic aromatic chemicals such as BTX (benzene, toluene, and xylene) and phenol. Producing these building blocks from lignin requires the selective hydrogenolysis of C-O bonds in phenols and aryl ethers, but their aromatic rings are also susceptible to hydrogenation. Using their new catalyst, the research group accomplished selective C-O bond hydrogenolysis without also cleaving the aromatic rings for the first time ever.
Professor Nozaki's research group employed hydroxycyclopentadienyl iridium complexes as catalysts under hydrogen (dihydrogen) at atmospheric pressure. Using these new catalysts, arenols (phenol derivatives) were successfully deoxygenated to afford the corresponding arenes. In addition, aryl methyl ethers were converted selectively to arenols after demethylation with dihydrogen using the same catalysts.
"This study shows the potential of our catalysts for application to the mass use of lignin as feedstock for production of basic aromatic chemicals for the chemical industry, instead of using fossil fuels," says Professor Nozaki. "Our final goal is to contribute to the creation of a sustainable society that makes efficient use of renewable resources."
More information: Nature Communications, 23rd February 2015. DOI: 10.1038/ncomms7296
Journal information: Nature Communications
Provided by University of Tokyo