What percent of Earth is water?

The Earth is often compared to a majestic blue marble, especially by those privileged few who have gazed upon it from orbit. This is due to the prevalence of water on the planet's surface. While water itself is not blue, water gives off blue light upon reflection.
For those of us confined to living on the surface, the fact that our world is mostly covered in water is a well known fact. But how much of our planet is made up of water, exactly? Like most facts pertaining to our world, the answer is a little more complicated than you might think, and takes into account a number of different qualifications.
In simplest terms, water makes up about 71% of the Earth's surface, while the other 29% consists of continents and islands.
To break the numbers down, 96.5% of all the Earth's water is contained within the oceans as salt water, while the remaining 3.5% is freshwater lakes and frozen water locked up in glaciers and the polar ice caps. Of that fresh water, almost all of it takes the form of ice: 69% of it, to be exact. If you could melt all that ice, and the Earth's surface was perfectly smooth, the sea levels would rise to an altitude of 2.7 km.
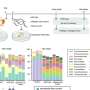
Aside from the water that exists in ice form, there is also the staggering amount of water that exists beneath the Earth's surface. If you were to gather all the Earth's fresh water together as a single mass (as shown in the image above) it is estimated that it would measure some 1,386 million cubic kilometers (km3) in volume.
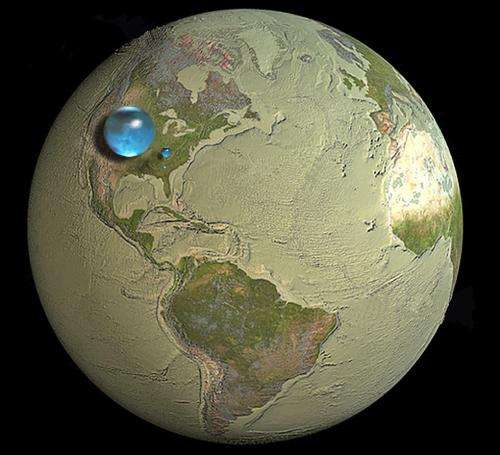
Meanwhile, the amount of water that exists as groundwater, rivers, lakes, and streams would constitute just over 10.6 million km3, which works out to a little over 0.7%. Seen in this context, the limited and precious nature of freshwater becomes truly clear.
But how much of Earth is water—how much water contributes to the actual mass of the planet? This includes not just the surface of the Earth, but inside as well. Scientists calculate that the total mass of the oceans on Earth is 1.35 x 1018 metric tons, which is 1/4400 the total mass of the Earth. In other words, while the oceans cover 71% of the Earth's surface, they only account for 0.02% of our planet's total mass.
The origin of water on the Earth's surface, as well as the fact that it has more water than any other rocky planet in the Solar System, are two of long-standing mysteries concerning our planet.
Not that long ago, it was believed that our planet formed dry some 4.6 billion years ago, with high-energy impacts creating a molten surface on the infant Earth. According to this theory, water was brought to the world's oceans thanks to icy comets, trans-Neptunian objects or water-rich meteoroids (protoplanets) from the outer reaches of the main asteroid belt colliding with the Earth.

However, more recent research conducted by the Woods Hole Oceanographic Institution (WHOI) in Woods Hole, Massachusetts, has pushed the date of these origins back further. According to this new study, the world's oceans also date back 4.6 billion years, when all the worlds of the inner Solar System were still forming.
This conclusion was reached by examining meteorites thought to have formed at different times in the history of the Solar System. Carbonaceous chondrite, the oldest meteorites that have been dated to the very earliest days of the Solar System, were found to have the same chemistry as those originating from protoplanets like Vesta. This includes a significance presence of water.
These meteorites are dated to the same epoch in which water was believed to have formed on Earth—some 11 million years after the formation of the Solar System. In short, it now appears that meteorites were depositing water on Earth in its earliest days.
While not ruling out the possibility that some of the water that covers 71 percent of Earth today may have arrived later, these findings suggest that there was enough already here for life to have begun earlier than thought.
More information: water.usgs.gov/edu/earthhowmuch.html
Provided by Universe Today