New techniques inspired by nature for accelerated aging and chemical extraction of mineral ores
Nature writ large is filled with examples of transformative chemical reactions that take place in the solid state—minerals found deep underground or on the surface of the earth that undergo chemical reactions over time when they are subjected to the right mix of environmental conditions.
The same cannot always be said for synthetic chemistry at the industrial scale. Factories that make pharmaceutical drugs and ore processing plants where precious metals are extracted from mined minerals tend to rely not on solids but solution chemistry—mixing salts or rocks with large volumes of often highly corrosive or toxic liquids to achieve chemical reactions and extract useful products. Those solvents, besides being expensive, often corrosive, and sometimes toxic to the environment, may be dangerously volatile—even explosive.
Now a team of researchers led by Tomislav Friščić, a professor at McGill University, is developing new approaches to chemical synthesis and mineral processing based on solid-state chemistry—and inspired by examples from nature.
At the 23rd Congress and General Assembly of the International Union of Crystallography, held August 5-12, 2014 in Montreal, Friščić will describe some of his unconventional approaches and how they promise better, safer and far less expensive methods for extracting metals from mineral ores as well as for the scalable synthesis of pharmaceutical drugs.
"You can use solid-state chemistry to make these materials much easier and cheaper, without the use of aggressive solvents or high temperatures," Friščić said.
Some of his favorite laboratory techniques include pounding materials with steel balls and leaving reactants to sit for days in swampy air. "We are trying to mimic what nature does," he said.
Accelerated Aging—A Lesson from Seagulls
Minerals are generally more or less inert materials—think of a handful of stones—and a whole industry exists for extracting useful ores from minerals taken from mines. But ore extraction usually relies on methods that involve mixing the minerals with massive amounts of volatile solvents at high temperatures.
One of the processes Friščić and his colleagues are seeking to mimic is the accelerated aging of minerals, a methodology inspired by weathering processes which occurs naturally in many places on Earth where the right conditions exist, such as in rocky coastal areas.
Drivers enjoying a wending ride along Highway 1 in California this summer can see examples of accelerated aging in the surf-battered rocks dotting the Pacific coast. Covered for much of the day with gulls and other birds, these rocks appear to be splattered with white paint because the mineral composition of rocky surfaces change due to the aging brought about by organic compounds in the bird poop mixed with the moist ocean air and the heat-baking sun.
Inspired by that picture, Friščić has developed simple methods in the laboratory for mimicking this natural process by exposing mixtures of reactants to selected mild conditions of humidity and temperature—methods that hold great promise for ore extraction and other applications that would not rely on dangerous solvents and would use far less energy, he said.
In Montreal, he will describe some of the initial experiments in which he mixed zinc with an organic compound derived from oxalic acid, left them at room temperature at high humidity for one week and derived from this 10-20 grams of microporous metal-organic frameworks—valuable materials for applications like carbon sequestration, gas sensing or solar panel production.
"I sometimes call accelerated aging the lazy man's chemistry because you can induce chemical changes without the input of a lot of external energy," he said.
Milling—An Ancient Process Yielding New Results
Another approach Friščić and his colleagues are developing is based on physically milling materials the way people have done since ancient times using a mortar and pestle.
According to research by Laszlo Takacs at the University of Maryland, Baltimore County, the first recorded example of a chemical process involving milling was practiced more than 2,000 years ago when stones of the mineral cinnabar were ground with vinegar in copper vessels to extract mercury.
Similar processes for triggering reactions and extracting useful chemicals could be implemented on industrial scales today, Friščić said, if we better understood how the milling process works.
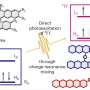
Asking that question, Friščić and a team of colleagues from Croatia, UK and Germany recently sought to get a better picture of what happens inside a shaker ball mill, a rapidly oscillating container in which chemical reactions are induced by pulverizing material with falling steel balls. The team obtained the support of the European Synchrotron Radiation Facility to bombard a ball mill with high energy X-rays as it mixed metal oxides and organic molecules together.
Nobody has ever applied this type of analysis to a milling process before, and it showed that as the grinding occurred, short-lived and previously unknown chemical intermediates were created in the process, some lasting only for a few seconds.
Understanding what these intermediates are and how they can be stabilized or otherwise exploited holds great promise for discovering new milling procedures on the industrial scale that will increase the speed, decrease the cost and eliminate the toxic waste of traditional solvent-based processes, Friščić said.
"Developing methods of solvent-free synthesis today is a bit like developing organic chemistry in the 1830s," Friščić said. "There is a lot of testing and detective work while trying to connect the dots. That provides particular pleasure."
Provided by American Institute of Physics