Finding the sweet spot for cartilage formation

Joint injuries often fail to mend properly when not given assistance. In particular, cartilage exhibits a poor capacity for self-repair. It is possible to stimulate regeneration by implanting synthetic scaffolds loaded with cartilage-forming cells (known as chondrocytes) at the site of an injury. However, the quality of the repair depends on the extent to which healthy cartilage can form on a given scaffold.
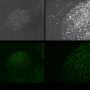
Now, researchers led by Motoichi Kurisawa of the A*STAR Institute of Bioengineering and Nanotechnology in Singapore have devised an optimized formula to encourage proper regrowth of cartilage. Kurisawa's group has worked extensively with polymers known as hydrogels (see image), whose stiffness strongly depends on the conditions under which they are synthesized. This property enabled the researchers to explore the extent to which the hydrogel stiffness affects cartilage formation. "Research indicates that cells that have adhered to a solid substrate are able to sense mechanical stimuli, and that this can affect many important physiological processes in a way similar to biochemical signals," says Kurisawa.
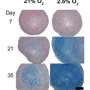
Ideally, joints should be enriched in smooth hyaline cartilage rather than tough and dense fibrocartilage, and the researchers suspected that stiffer hydrogel scaffolds might promote increased fibrocartilage formation. To test this idea, they synthesized three different kinds of gelatin–hydroxyphenylpropionic acid (Gtn–HPA) hydrogels, which they classified as having low, medium or high stiffness. Chondrocytes generally survived well and proliferated when seeded onto all three scaffolds, but they produced cartilages of considerably different qualities.
Chondrocytes cultivated on Gtn–HPA-high produced the highest density of cartilage, but this tended to be primarily fibrous in nature, whereas cells grown on Gtn–HPA-low produced only low levels of cartilage. However, the medium-stiffness scaffold proved well suited to the production of hyaline cartilage, facilitating efficient tissue repair after implantation in a rabbit model of joint injury. After four weeks, these animals showed robust hyaline cartilage growth; in contrast, recipients of low- and high-stiffness scaffolds exhibited only limited repair or dense fibrous tissue formation, respectively. Importantly, the Gtn–HPA-medium scaffold also readily dissolved after implantation.
"Our findings highlight the importance of incorporating considerations of hydrogel stiffness into the design of scaffolds intended for cartilage tissue engineering," says Kurisawa. Based on the optimal 'middle ground' identified in this study, he and his colleagues are now hopeful that they can demonstrate the same level of safety and efficacy in large animal models as the next step toward moving to the clinic.
More information: Wang, L.-S., Du, C., Toh, W. S., Wan, A. C. A., Gao, S. J. & Kurisawa, M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 35, 2207–2217 (2014).
Journal information: Biomaterials