Snapshots of atoms make it into physics textbooks
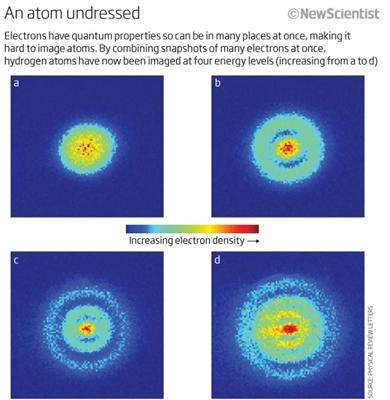
Physicist Aneta Stodólna captured the electron positions of hydrogen atoms on camera for the very first time. The snapshots from her quantum-style microscope gained worldwide attention and even made it into physics textbooks. On 20 June 2014, Aneta Stodólna will obtain her doctorate from Radboud University.
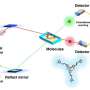
'Other physicists already dreamed up the technique we used over 30 years ago', Stodólna explains, 'but we were the first to actually make a functional quantum microscope for hydrogen atoms and take pictures with it. That way we can determine the locations of an atom's electrons and with that, the atom's properties. It was a real challenge though, at some point I thought we would never get the set-up to work. I was so relieved when we found the right signal after months of puzzling. I knew that this result was really, really big.
Proof of principle
Stodólnas experimental set-up made use of 'velocity map imaging', a technique originally invented in Nijmegen by professor David Parker, experimental physicist at the molecular and laser physics department. He explains: 'These images finally visualized of what we thought we knew about the hydrogen atoms wave function. Or actually, what we hoped we knew. Anetas results are a beautiful step forward in physics, they are the most direct and convincing proof of principle of an atom's wave function I've seen so far.'
Atom snapshots go worldwide
The images made in into Physics Review Letters in May 2013 and gained worldwide attention from physicists and the general public afterwards. PhysicsWorld magazine chose the publication as one of the top 10 breakthroughs in physics in 2013 and Stodólnas work is already included in several textbooks.
More information: Doctoral defence: Taking snapshots of atomic wave functions with a photoionization microscope: www.ru.nl/@936142/taking-snapshots/
Publication: Hydrogen Atoms under Magnification: Direct Observation of the Nodal Structure of Stark States , Phys. Rev. Lett. 110, 213001 – Published 20 May 2013 : journals.aps.org/prl/abstract/ … ysRevLett.110.213001
Journal information: Physical Review Letters
Provided by Radboud University Nijmegen