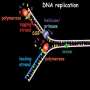
'Cowcatcher' enzyme fixes single-strand DNA
Every time one of your cells divides, it exposes its most essential component to great danger: its genome, the sum total of all its genetic information, embodied in the double-stranded helix of DNA. Prior to cell division, this DNA splits into two single strands, each bearing sequences of biochemical bases that form templates for the genomes of the daughter cells. These single strands are particularly vulnerable to assaults by reactive oxygen species—toxic byproducts of respiration—that could cause changes in the genetic information they contain.
Left unchecked, such mutations would quickly add up, producing cells riddled with genetic errors—a recipe for DNA-damage linked disorders such as cancer, aging and neurodegenerative diseases. However, through evolution, mammalian cells have developed a way to repair damaged bases in the single-stranded genome. Now University of Texas Medical Branch at Galveston researchers have figured out how this process works, publishing their results this week in the Proceedings of the National Academy of Sciences.
To understand the UTMB researchers' work, it helps to picture DNA strand separation during replication as analogous to the opening of a zipper. As the "zipper" opens, it exposes strings of four uniformly spaced bases attached to each single strand of DNA. Not far behind, each of these strands is straddled by an advancing "replication complex" of proteins busily copying the single strand back into double strand. The single-strand repair problem is located between these new double strands and the opening zipper, where the DNA is most susceptible to damage and removal of a damaged base would cause the strand to break.
The UTMB scientists' work centers on an enzyme called NEIL1, which scientists knew recognized single-stranded DNA and also knew was associated with the replication complex. In a series of in vitro experiments, the researchers determined that NEIL1 actually rides in front of the replication complex, scouting for single-strand DNA damage.
"As soon as it encounters the base damage, NEIL1 binds to the damage site and flags it, and replication cannot continue," said UTMB assistant professor Muralidhar Hegde, the lead author on the paper. "The replication machinery stalls and then regresses, and the two strands come back together which allows repair of the damaged base in duplex DNA, replacing the damaged base with the appropriate normal base."
Then the "DNA zipper" begins opening again.
"The replication machinery comes back and it continues," Hegde said. "So we have NEIL1 both looking at what is ahead and signaling to the back."
The paper's senior author, professor Sankar Mitra, has his own analogy for the role of NEIL1: he calls it a "cowcatcher" enzyme, comparing it to the structure on the front of an early steam locomotive used to clear animals or debris off the track. "Basically, the replication train is coming and the cowcatcher is in front to see if any damage is present," Mitra said. "If it finds such damage, the train moves backward, you repair it, and the train starts moving forward again. Of course, that's a simplification of a very complex chemical reaction—an exquisitely controlled and regulated process, and one that's very important to maintaining the integrity of the genome."
More information: Prereplicative repair of oxidized bases in the human genome is mediated by NEIL1 DNA glycosylase together with replication proteins, www.pnas.org/cgi/doi/10.1073/pnas.1304231110
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Texas Medical Branch at Galveston