Experiments validate quantitative model predictions for cell activation dynamics
(Phys.org)—In biological research, a typical process would be to conduct experiments and then analyze the collected data to reach conclusions. A new model-based analysis approach from computational biology scientists at Pacific Northwest National Laboratory demonstrates that integrating computations into experimentation enables researchers to obtain more robust quantitative information about cell kinetics.
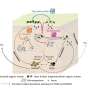
The process uses a computational model to generate predictions about the dynamics of cell receptors in different cellular compartments. The model predicted that the time scales of receptor (de)activation kinetics on the cell surface and the interior compartments were comparable. This finding initially appeared contrary to what would have been expected based on existing literature; however, follow-up experiments validated that the computational predictions were actually correct.
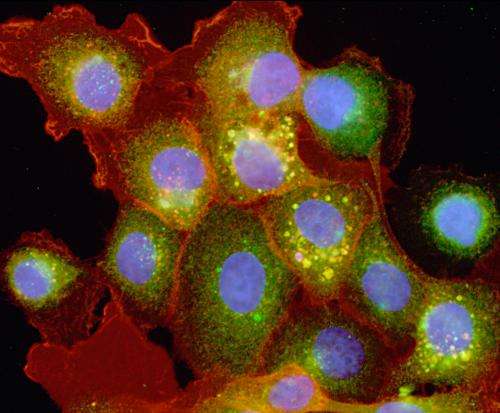
The work is featured on the November cover of Molecular BioSystems.
In human cells, signaling (i.e. telling the cell what to do such as releasing hormones or regulating a cell cycle) is initiated by external cues, and cell receptors facilitate the relay of the received information to regulatory elements in the cell. Since knowing their differential cellular signaling patterns can provide clues about the potential effectiveness of drug responses and treatment strategies, determining whether surface and internal receptors function the same way is important. Current data indicate that surface and internal cell receptors may have differing response and activation kinetics, that is, they may respond differently when exposed to the same stimulus. But using the new model, PNNL scientists predicted that the receptors would actually have similar deactivation kinetics. Laboratory measurements of receptor phosphorylation levels—which indicate the level of their activity—showed that, in fact, deactivation rates were roughly the same for both the internal and surface receptors.
The PNNL team developed mathematical models for epidermal growth factor receptor (EGFR) signaling to predict how the activated receptors were spatially distributed within cells. In order to maintain simplicity, model development and the design of future experiments were integrated from the beginning. The model was constructed to predict the receptor phosphorylation levels in a cell under specific conditions for the immediate-to-short (minute-to-hours) response durations. The team then performed the validation laboratory tests to determine the actual EGFR phosphorylation levels in cells, both for all the cell receptors and for only the internal receptors. The difference between these two measurements provided the information about the surface receptors.
When receptors receive a cue to initiate a signal, the signal is transmitted "downstream" along signaling pathways to trigger the next phase of activity. Such signal propagation occurs through transient protein-protein interactions and may involve a large number of associated proteins. The PNNL team is now investigating how different ways for initiating a signal translate into different response patterns at the level of downstream proteins. Additionally, they are looking at how differential signaling, i.e., variations in signaling patterns when conditions change, may be regulating cell decision-making, thus resulting in different responses based on the cell's observed properties.
More information: Shankaran, H. et al., Integrated Experimental and Model-based Analysis Reveals the Spatial Aspects of EGFR Activation Dynamics, Molecular BioSystems 8(11):2868-2882. DOI:10.1039/C2MB25190F.
Provided by Pacific Northwest National Laboratory