Researchers develop novel technique for early detection of misfolded protein

(Phys.org) -- University of Delaware assistant professor David W. Colby is co-author of a paper in the March 23 issue of the Journal of Biological Chemistry that suggests protein misfolding may occur early in the pathogenesis, or development, of Huntington’s disease.
Huntington’s disease (HD) is one of several neurological diseases, such as Alzheimer’s disease or prion disease, associated with proteins that fold into abnormal structures. HD is characterized by progressive motor impairment, cognitive decline and behavioral abnormalities, and ultimately death.
The researchers developed a novel technology, called an amyloid seeding assay (ASA), to detect the misfolded protein, huntingtin, in laboratory mice at 11 weeks of age, more sensitively than traditional histology methods which don’t reveal large inclusions until much later in the pathogenic process, about 78 weeks.
According to Colby, the ASA takes advantage of the biophysical tendency of isolated misfolded huntingtin to act as a “seed” for the conversion of a monomeric polyglutamine peptide to a misfolded form, known as an amyloid fiber.
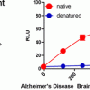
This results in the formation of additional amyloid protein, essentially amplifying the amount of misfolded protein in the sample. The amyloid can then be detected with the dye Thioflavin T and measured by a fluorescent detector.
“Alzheimer’s disease and prion disease brain tissue subjected to the same purification procedure did not do so, demonstrating the specificity of the ASA,” the paper states.
“Testing of experimental therapies is slow and expensive, given the time it takes for a misfolded protein to appear in a form detectable by traditional methods. We believe that the ASA can speed up this initial testing process and push promising therapies to clinical trial faster,” Colby said.
Provided by University of Delaware