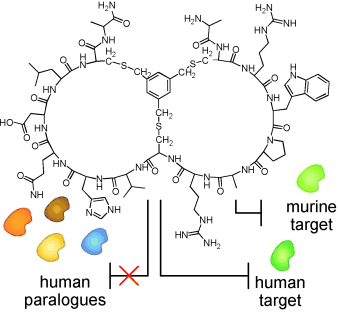
Bicyclic peptides with optimized ring size inhibit human plasma kallikrein and its orthologs while sparing paralogous pr
(Phys.org) -- New drug candidates require testing in animal models prior to approval for clinical use. A recently developed antagonist based on a bicyclic peptide inhibited the human serine protease plasma kallikrein potently and selectively. However, the inhibitor was ‘too’ selective, not inhibiting murine plasma kallikrein which prevented its testing in animal models. Researchers led by Christian Heinis have developed bicyclic peptides that inhibit both human and murine plasma kallikrein, but not any paralogous proteases; their work is reported in ChemMedChem.
"We compared structural models of target (and related non-target) proteases to identify conserved regions in the vicinity of the active site, and modulated the loop size of our libraries of peptide macrocycles accordingly," says Heinis on behalf of his team at the Swiss Federal Institute of Technology in Lausanne and colleagues John Tite (Bicyclic Therapeutics) and Greg Winter (Laboratory of Molecular Biology; both Cambridge, UK). "From these libraries, we were able to isolate potent bicyclic peptide inhibitors of human, rat and monkey plasma kallikrein that do not inhibit related human serum proteases."
Based on structural considerations, the authors hypothesize that the length of the peptide loops may influence specificity. By modulating the loop size of the peptide macrocycles, they succeeded in selecting potent human plasma kallikrein inhibitors with the desired specificity profile. Heinis states further that "...the bicyclic peptides combine key qualities of antibody therapeutics (high affinity and specificity) and advantages of small-molecule drugs and may offer an attractive format for the development of therapeutics." The authors conclude that this strategy will likely facilitate the use of peptide macrocycles in animal models, while avoiding unwanted off-target activities in the clinic.
More information: Christian Heinis, Bicyclic Peptides with Optimized Ring Size Inhibit Human Plasma Kallikrein and its Orthologues While Sparing Paralogous Proteases, ChemMedChem, dx.doi.org/10.1002/cmdc.201200071
Journal information: ChemMedChem
Provided by Wiley