New method to clean and treat polluted water for extraction of chemicals

Scientists in Poland have discovered that it is easy to clean and treat polluted water for extraction of valuable chemicals, such as those used in the production of drugs. The upshot of this is that the use of neither plants nor factories is required; only the Sun and a 'magic' powder are needed to get the job done. The study is presented in the journal Bioresource Technology.
Researchers from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw say this nearly alchemic transformation is made possible by photocatalysts, which are the subject of their investigation.
Our planet contains areas where water is highly polluted by organic chemicals from industrial wastes. Thanks to the work carried out by the Polish team, we know that the biomass can be successfully transformed into chemicals and fuel. The photocatalysts facilitate the transformation of polluted water into clean water. Another advantage is that specialised plants are not needed, as the transformation occurs under conditions that are commonly met in nature.
The chemical reaction is supported by a catalyst, which helps accelerate the course. Once the reaction is finalized, the catalyst recovers. Experts say that in typical catalytic processes, the catalysts are activated at high temperatures, around several hundreds degrees Celsius, and usually at very high pressure.
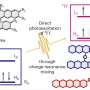
"Photocatalysts studied by us differ in many respects from traditional catalysts,' says Dr. Juan Carlos Colmenares from the IPC PAS. 'They are activated by light, and the temperature has no significant effect here."
The reactions that get support from the photocatalysts occur at good exposure to sun rays, with the temperature at around 30o Celsius and atmospheric pressure being normal. The conditions, say the researchers, are occurring naturally all year round in many equatorial countries.
The photocatalysts in question are solids based on titanium dioxide, TiO2. The catalysed reaction occurs in a liquid containing organic pollutants. Once the reaction is completed, the catalyst can be isolated almost without losses and used again, according to the team.
"My work resembles somewhat alchemy," Dr. Colmenares comments. "I take a 'magic' powder, pour it into polluted water, stir and expose to the Sun. After several hours, I get clean water plus chemicals that can be used to make useful things, for instance drugs."
Since the 1960s, researchers around the world have been investigating the photochemical degradation of pollutants, and they have collected chemical compounds by intensive ultraviolet (UV) irradiation.
The work conducted by the IPC PAS team targeted a reaction that can occur without the use of specialized equipment. The degradation of biomass that stops at a precisely defined stage was also targeted. The researchers generated carboxylic acids with titania-based photocatalysis. These acids are used in the pharmaceutical and food industries, for example.
Says Dr. Colmenares: "In laboratory conditions, the reactions of the biomass with participation of photocatalysts are promising already now. In this year we are going to attempt the first tests in the pilot biochemical photoreactors at the University of Cordoba, Spain. The reactions will occur there in liquids with volumes measured in tens of litres."
More information: Colmenares, J.C. et al. (2011) 'High-value chemicals obtained from selective photo-oxidation of glucose in the presence of nanostructured titanium photocatalysts', Bioresource Technology, 102(24), 11254. doi: 10.1016/j.biortech.2011.09.101
Provided by CORDIS