Method could help carbon nanotubes become commercially viable
Carbon nanotubes are intriguing new materials which have been highly touted for their exceptional mechanical, thermal, optical and electrical properties.
Researchers worldwide are striving to apply these nanostructures in electronics, high-resolution displays, high-strength composites and biosensors. A fundamental problem relating to their synthesis, however, has limited their widespread use.
Current methods for synthesizing carbon nanotubes produce mixtures of tubes that differ in their diameter and twist. Variations in electronic properties arise from these structural differences, resulting in carbon nanotubes that are unsuitable for most proposed applications.
Now, a new method developed at Northwestern University for sorting single-walled carbon nanotubes promises to overcome this problem. The method works by exploiting subtle differences in the buoyant densities of carbon nanotubes as a function of their size and electronic behavior. The results will be published online Wednesday, Oct. 4, in the inaugural issue of the journal Nature Nanotechnology (October 2006).
"Carbon nanotubes, because of their ultra-small size and excellent materials properties, have excited the scientific community for the last decade," said Mark Hersam, professor of materials science and engineering at Northwestern's McCormick School of Engineering and Applied Science, who led the research team.
"However, due to their inherent heterogeneity, they have not yet realized their full commercial potential," he said. "A scalable and economical method for producing monodisperse carbon nanotubes will enable the proposed applications for these nanomaterials to be explored at an industrially relevant scale."
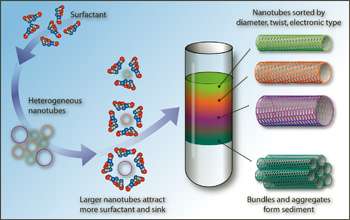
Using the Northwestern method, carbon nanotubes first are encapsulated in water by soap-like molecules called surfactants. Next, the surfactant-coated nanotubes are sorted in density gradients which are spun at tens of thousands of rotations per minute in an ultracentrifuge. By carefully choosing the surfactants utilized during ultracentrifugation, the researchers found that carbon nanotubes could be sorted by diameter and electronic structure.
As a part of their study, the researchers demonstrated the fabrication of electrical devices that displayed either semiconducting or metallic behavior, depending on the sorted nanotubes used. The researchers also maintain that their technique can be translated to an industrial scale.
"The technique is especially promising for commercial applications," said Hersam, "because large-scale ultracentrifuges have already been developed and shown to be economically viable in the pharmaceutical industry. We anticipate that this precedent can be straightforwardly translated to the production of monodisperse carbon nanotubes."
Source: Northwestern University





















