February 25, 2015 report
Study demonstrates an electronic switch based on stereoisomerism
As devices get smaller and smaller, scientists are running up against limits to how small one can feasibly construct a circuit using bulk materials. Molecular circuits offer a possible solution to overcoming these size constraints, and have led to a growing field merging chemistry with electronics.
One study from lead author Timothy A. Su and a team from Columbia University report the first of its kind single-molecule switch with two distinct conductance phases that is based on the molecule's two stereoisomers. Their work appeared in Nature Chemistry.
Conductivity is based on the movement of electrons. Metals are highly conductive because electrons easily traverse through the material. Nonmetal molecules, such as alkanes, are also conductive, but are lower in conductivity than metals because electrons do not travel as easily through the sigma bonding network. However, these long-chain nonmetals are attractive for molecular circuitry because of their synthetic and geometric versatility. Oligosilanes offer a better option for electron mobility because of increased sigma delocalization along the Si-Si bonds, while also maintaining the synthetic and geometric versatility that makes alkanes attractive.
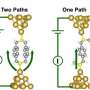
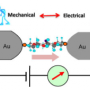
Su et al. tested various silanes (permethyloligosilanes) with methythiomethyl substituents on either end of the oligosilane molecule. They tested the conductance of [SiMe2]n where n represents from one to ten permethylsilanes. Conductance was tested using scanning tunneling microscope break junction, similar to attaching the terminal methylthiolmethyls to molecular-sized gold electrodes such that the molecule is bridged a Au-[SiMe2]n-Au fashion. Conductance was measured relative to the length of the oligosilane and relative to the distance between the gold STM tip and electrode, or as the oligosilane was systematically expanded and compressed between the two gold surfaces.
Results from testing the various lengths of oligosilanes showed decreased in conductivity as length of molecule increases. This "length-dependent conductance decay" is an expected property of long-chain nonmetals and has been observed in alkanes, as well.
However, unlike alkanes, in all of the oligosilanes there was an abrupt change from low to high conductance as the distance between the electrodes increases. One would expect the conductance to decrease as the distance between the gold tip and electrode increased. Additionally, this abrupt change was by a factor of two for all oligosilanes, regardless of length of the silicon chain. The length of the low conductance plateau increased as length of oligosilane increased, but the length of the high conductance plateau was the same for all molecules, indicated that this state was due to a common feature in all of the molecules and was unrelated to the length of the oligosilane chain.
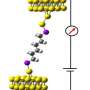
This distinctly two-state conductivity feature was likely due to the terminal dihedral angles formed by the Au-S-C-Si bonds since this feature was the same for all molecules. To confirm that the change in conductance was due to stereoelctronic effects, Su et al. conducted DFT analysis to determine the lowest energy conformation of their oligosilane at varying distances between two gold atoms. They used [Au-Si(4)-Au]2+ structure as their test molecule to mimic the electronic effects of the STM system. For this experiment, they began with gold atoms at a distance that would provide dihedral angles in an anti conformation without constraint and increased the distance between the gold atoms by 0.25 Angstrom increments.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
They found that the distance between the gold atoms plays a crucial role in molecular conformation and therefore on the conductivity of the oligosilane. During the low conductance state, the Me-S bond is antiperiplanar (Au-S bond is perpendicular) to the methylsilane bond, or in an anti conformation. At the transition to high conductance, the Me-S bond is perpendicular (Au-S bond is antiperiplanar) to the methylsilane bond, or in an ortho conformation.
The anti conformation overcomes steric strain, but the ortho conformation overcomes the mechanical strain from electrode separation. The anti conformation has Au-S orbitals that are perpendicular to the plane of the Si-Si bonds, hindering electron tunneling through the molecule, while the ortho conformation has Au-S orbitals that aligned on the same plane as the Si-Si bonds, allowing for greater electron mobility through the sigma bonding network.
Electrochemical switching occurs at a specific Au-Au distance for each of the oligosilanes, and conductance changes in real time relative to distance. Furthermore, the molecular switch has two discrete conductance states, as opposed to a third transitional state. While there is a point when one terminal dihedral bond is in an ortho conformation and the other is an anti conformation, the conductance remains in the low state until both bonds are in the ortho conformation, making this a true binary switch based on stereoelectronic effects.
More information: Nature Chemistry 7, 215–220 (2015) DOI: 10.1038/nchem.2180
Journal information: Nature Chemistry
© 2015 Phys.org