January 22, 2015 report
Researchers create stable gold(III) catalysts via oxidative addition of a carbon–carbon bond
(Phys.org)—A team of researchers working at the University of California, has found a way to create stable gold(III) catalysts using oxidative addition of a carbon–carbon bond. In their paper published in the journal Nature, the team describe how they developed a technique that allowed for a carbon–carbon bond split at room temperature by an Au(I) complex that generated a stable Au(III) cationic complex. Christopher Kourra and Nicolai Cramer with École Polytechnique Fédérale de Lausanne offer a News & Views piece in the same journal issue describing the work by the team at UC.
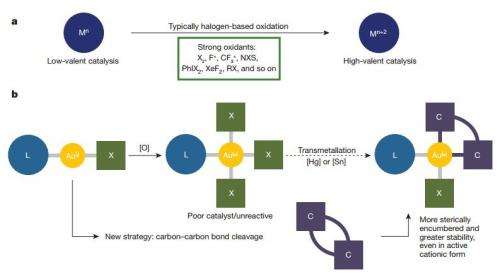
As Kourra and Cramer note, a major challenge for chemists in the organic field is to synthesize structurally complex target molecules selectively, allowing for the development of pharmaceuticals and other valuable materials. One way to do so is via catalysis, which has proven successful in a number of ways. There still remains one area that has met with little success, however, catalysis with transition metals which exist in high oxidation states—Au(III) is one. In this new effort, the researchers have found a way to overcome the two main obstacles (poor stability and the difficulty of getting to the oxidation state via so-called "mild" techniques) researchers have faced in the past.
The team's technique consisted of inserting an existing Au(I) catalyst (biphenylene) into a carbon-carbon bond, without using any strong oxidants. The results was an Au(III) catalyst which had a biphenylene ligand. They note that the reaction occurred at room temperature, making the process relatively simple and inexpensive. Next, the group converted their catalyst into a precursor (stable) compound for storage until a reaction is needed. The researchers then put the ligand to good use—to guide oxygen atoms on substrates into groups that would bind to the metal, offering in effect, a protective shield of sorts against complementary reactions.
The researchers tested their catalyst in six different types of reactions and report good product yields, which meant that their technique represents a major breakthrough in the field. They noted also that it opens the door to using Au(I) and Au(III) together, offering the benefits of both, which had not been done before. They next plan see how well their technique works with other compounds.
More information: Stable gold(III) catalysts by oxidative addition of a carbon–carbon bond, Nature 517, 449–454 (22 January 2015) DOI: 10.1038/nature14104
Abstract
Low-valent late transition-metal catalysis has become indispensable to chemical synthesis, but homogeneous high-valent transition-metal catalysis is underdeveloped, mainly owing to the reactivity of high-valent transition-metal complexes and the challenges associated with synthesizing them. Here we report a carbon–carbon bond cleavage at ambient conditions by a Au(I) complex that generates a stable Au(III) cationic complex. In contrast to the well-established soft and carbophilic Au(I) catalyst, this Au(III) complex exhibits hard, oxophilic Lewis acidity. For example, we observed catalytic activation of α,β-unsaturated aldehydes towards selective conjugate additions as well as activation of an unsaturated aldehyde-allene for a [2 + 2] cycloaddition reaction. The origin of the regioselectivity and catalytic activity was elucidated by X-ray crystallographic analysis of an isolated Au(III)-activated cinnamaldehyde intermediate. The concepts revealed suggest a strategy for accessing high-valent transition-metal catalysis from readily available precursors.
Journal information: Nature
© 2015 Phys.org




















