New analysis explains collagen's force
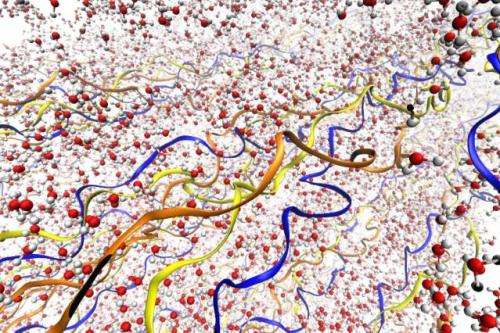
Research combining experimental work and detailed molecular simulations has revealed, for the first time, the complex role that water plays in collagen—a protein that is a component of tendons, bone, skin and other structural tissues in the body.
The new analysis reveals an important mechanism that had never been observed before: Adding even small amounts of water to, or removing water from, collagen in tendons can generate surprisingly strong forces, as much as 300 times stronger than the forces generated by muscles. The findings are reported this week in the journal Nature Communications by researchers at MIT and the Max Planck Institute for Colloids and Interfaces in Germany.
"We don't really know the physiological role of water" in the human body's collagen-based tissues, explains Professor Markus Buehler, head of MIT's Department of Civil and Environmental Engineering and a co-author of the paper. "Here we show that it can develop significant forces, especially in tendons, which are thought of as a passive material."
One of the challenges in previous studies has been that natural biological samples are all different, Buehler says—so trying to determine the underlying causes of variability is tricky. In the new work, the team was able to study the same samples under a variety of conditions, enabling them to probe the causes of variations.
Then, these same materials were analyzed using an atom-by-atom computer model that can simulate the structure down to the level of individual molecules, providing a detailed view of the underlying mechanisms. The molecular simulations, carried out at MIT by Buehler and postdoc Shu-Wei Chang, matched and complemented the experimental results observed by the team members in Germany, led by Professor Peter Fratzl.
The real and the virtual
"We can look at the same scale, the same phenomena, in both experiments and simulations," Buehler says. "We're very excited by the results," he adds, because the simulations make it possible to view "mechanisms that you cannot easily measure" in physical experiments.
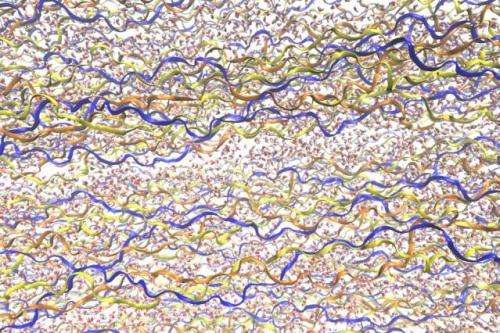
Chang explains that based solely on the experimental data, there are two possible explanations for the behavior of the tendon material—but the simulations reveal that one of these explanations is infeasible. The researchers showed that within the complex structure of the protein chains that make up tendon collagen, the addition of water causes some parts of the molecule extend, and others to shrink.
The balance between the two mechanisms determines whether there is an overall shrinkage or extension of collagen molecules, Chang says—but overall, when water is removed from the molecular structure, it shrinks.
Powerful forces
The pull of that contraction is startlingly large: The force the drying process exerts "is almost three orders of magnitude greater than the forces generated by muscles," Chang says. It remains to be understood what, if any, role those forces play in normal biological functioning.
Whatever the function of this process in the body, it could potentially be harnessed through tissue engineering and used for other purposes, Buehler says: "We could use water as a driver, as one of the variables to control the material." Previously, he adds, "We would not worry about the amount of water in the material." But now it's possible to envision, for example, "a self-assembling system where water is regulating the process."
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
Scientists might also be able to get the contrary characteristics of different biological materials to work in concert. For example, combining a material like spider silk—which actually shrinks with the addition of moisture—with a material like tendon collagen could yield a hybrid structure that doesn't expand or shrink as humidity varies, Buehler suggests, providing a very stable structure.
In addition, the methods used in this analysis, combining detailed molecular modeling with direct experimental observation, could also be applied to many other biological materials, Chang says.
Sandra Shefelbine, an associate professor of mechanical and industrial engineering at Northeastern University who was not connected with this research, says the MIT team's analysis "is an excellent example of how computational molecular models can be used in coordination with experiments to discover mechanisms at the atomic level." David Mooney, a professor of bioengineering at Harvard University, adds that "this is a very striking finding, as it reveals new aspects of the structure and function of collagen, and is likely to lead to many new studies."
Journal information: Nature Communications
Provided by Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.




















