December 9, 2014 report
What makes Champagne bubbly?
(Phys.org)—Just in time for the holidays, scientists have unraveled some of the chemistry behind the diffusion of CO2 molecules in a glass of Champagne. Among their findings, they discovered that ethanol is the main molecule (along with water) responsible for the value of CO2 diffusion coefficients in Champagne, and is therefore an essential molecule to better understand the CO2 bubble formation and growth in these beverages. Besides shedding light on the bubble dynamics and subsequent tasting sensations of Champagne, the results could also have applications for evaluating the diffusion of CO2 molecules in water/alcohol mixtures that are commonly used in physical chemistry.
The researchers, David A. Bonhommeau, et al., from the University of Reims Champagne-Ardenne, have published their findings in a recent issue of The Journal of Physical Chemistry Letters. The work was supported by the Bull company, experts in HPC (High-Performance Computing); and ANRT (the Association Nationale de la Recherche et de la Technologie).
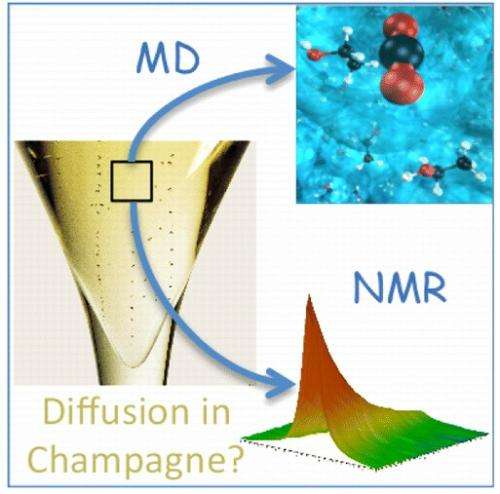
"The greatest theoretical significance of this work is to demonstrate, by extensive comparison with nuclear magnetic resonance (NMR) and viscometry measurements, that the diffusion and viscosity in supersaturated multicomponent liquids such as Champagnes can be investigated at various temperatures by standard molecular dynamics simulations," Bonhommeau told Phys.org. "Moreover, the temperature dependence of the CO2 diffusion coefficient is useful information to better apprehend the kinetics of CO2 release under standard tasting conditions, i.e., to better apprehend how long a Champagne or a sparkling wine keeps its so much sought-after effervescence."
As the scientists explain, previous research has shown that two phenomena are responsible for the emission of CO2 bubbles in sparkling beverages such as Champagne wines. The first phenomenon arises because these beverages are supersaturated with CO2, and CO2 emission occurs at the interface between a supersaturated aqueous solution and a gas phase (the air above the glass).
The second phenomenon responsible for CO2 bubble emission is effervescence, which refers to the formation of bubbles from tiny gas pockets trapped within immersed particles, such as cellulose fibers, crystals, or even within scratches or etchings on the glass surface. When the radius of the gas pocket trapped within the particle or scratch exceeds a critical size (about 0.2 µm at the opening of a Champagne bottle), dissolved CO2 can diffuse into the gas pocket and make the bubble grow. As the CO2 diffuses, many CO2 bubbles are released in the Champagne in the form of bubble trains.
To better understand this bubble formation process, the researchers in the current study investigated the interplay between CO2 and ethanol molecules in Champagne wines and, unlike in some previous research, took account of the influence of temperature on the CO2 dynamics. Their molecular dynamics simulations showed that CO2 diffuses more rapidly at higher temperatures, in agreement with their NMR spectroscopy measurements.
Their results also show that CO2 diffusion coefficients are about twice as large as ethanol diffusion coefficients. This is because, compared to CO2 molecules, ethanol molecules form at least five times more hydrogen bonds with the surrounding water molecules, making ethanol molecules less mobile. From this perspective, ethanol molecules and water molecules react to form a bonding network, while CO2 molecules can be considered a spectator species, having more freedom to float to the top.
Because the value of CO2 diffusion coefficients depends mainly on ethanol, the results suggest that there should be no major correlation between the taste of sparkling wines, which is mainly due to acids, sugars, and proteins, and the formation and growth dynamics of bubbles.
"For industrial companies, [our results show] that modifying the composition of Champagne wines—in which ethanol concentration is fixed to 12.5% v/v in champagnes—will probably have little effect on the formation and growth dynamics of bubbles that is believed to have an influence on tasting sensations," Bonhommeau said.
In addition, the researchers showed that the molecular dynamics simulations validate results from previous research. Specifically, the simulations show that an empirical method of determining the CO2 radius is sufficiently accurate to be used to approximate or evaluate diffusion coefficients or viscosities in liquids. This approach could be used to study a variety of transport phenomena in supersaturated solutions or water/alcohol mixtures, which are often used as solvents in chemistry research.
"The Stokes-Einstein relationship connecting the diffusion coefficient of a species and the viscosity of the liquid depends on the species hydrodynamic radius (the species being assumed spherical)," Bonhommeau said. "It is hard to exactly know the value of this effective radius. We proved in the paper that using the rms (root mean squared) distance of the species atoms with respect to its center of mass leads to a reliable first guess for this radius, at least for relatively small molecules. This means that researchers running molecular dynamics simulations could straightforwardly evaluate diffusion coefficients (resp. viscosity) from the knowledge of viscosity (resp. diffusion coefficients) by only applying the Stokes-Einstein formula, although this formula is only rigorously valid for more macroscopic systems."
Finally, the same approach used to model Champagne used here could be applied to many other applications.
"Although Champagne is a multicomponent liquid by nature (that is, composed of a number of different molecules), CO2 diffusion can be simply modeled by applying methods commonly used for binary mixtures," Bonhommeau said. "Such an approach could be extended to the investigation of the diffusion of small molecules in other supersaturated liquids such as brines (i.e., water in oceans) or maybe in amorphous solids (molecules trapped in ice for atmospheric or astrochemical applications). It is sometimes believed that convergence may be hard to reach with such simple models, but we proved, by comparing our results to NMR measurements, that excellent agreement can be achieved by using standard molecular dynamics simulations provided that suitable water models and appropriate techniques for evaluating diffusion coefficients are employed."
More information: David A. Bonhommeau, et al. "Unveiling the Interplay Between Diffusing CO2 and Ethanol Molecules in Champagne Wines by Classical Molecular Dynamics and 13C NMR Spectroscopy." The Journal of Physical Chemistry Letters. DOI: 10.1021/jz502025e
Journal information: Journal of Physical Chemistry Letters
© 2014 Phys.org