Scientists model molecular movement within narrow channels of mesoporous nanoparticles
Scientists at the U.S. Department of Energy's Ames Laboratory have developed deeper understanding of the ideal design for mesoporous nanoparticles used in catalytic reactions, such as hydrocarbon conversion to biofuels. The research will help determine the optimal diameter of channels within the nanoparticles to maximize catalytic output.
Porous nanoparticles are lab-created tiny spheres that incorporate even tinier parallel channels or pores. In catalytic processes, each channel within a particle is lined with catalytic sites that convert a reactant to a product. What's appealing about porous nanoparticles is that the walls of the pores provide significant surface area to support catalytic sites within a super-small sphere. And, as one might expect, the more pores, the more surface area, the better the catalytic reaction.
"Downside is that when the catalytic sites are within narrow pores, as is the case with mesoporous nanoparticles, the entire reaction, including the movement of reactants and products has to happen within the narrow channel," said Jim Evans, a scientist at Ames Laboratory who led the research. "Just like anyone who has found themselves trying to move around a crowded grocery store aisle, it's not always so easy to move past others in a very narrow space."
So, the optimal design for mesoporous nanoparticles hinges on the diameter of the individual channels: narrow enough to fit as many pores in each particle as possible to maximize the number of catalytic sites—but wide enough for catalytic products and reactants to easily squeeze by each other and efficiently complete the reaction. To determine this "sweet spot" for channel diameter, scientists must better understand how molecules move past each other within the channel.
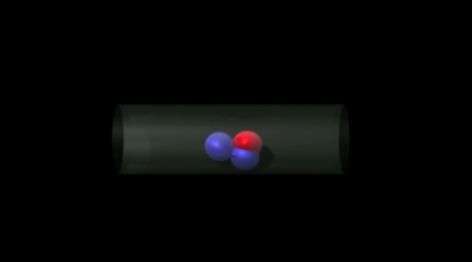
"In particular, it is helpful to know how often a nearby pair of reactant and product molecules pass by each other versus how often they separate from one another. Determining this 'passing probability' for different pore diameters and various relevant molecular shapes helps determine just how narrow channels can be before the catalytic output is reduced," said Evans.
Evans and his collaborators ran millions of simulations trials for pairs of sphere-shaped molecules and pairs of more irregularly-shaped molecules. These enabled precise determination of passing probability behavior for narrow pores.
"However, simulation becomes demanding and results less reliable for realistic irregular-shaped molecules with many rotational degrees of freedom. Also, just running simulations does not necessarily provide a deep understanding as to what features control behavior," said Evans.
So, he brought together expertise at Ames Laboratory in both theoretical chemistry and applied mathematics to determine and implement the best theoretical and modeling tools to get more reliable results and deeper insights into how the passing probability falls to zero as the channel size narrows.
"It was the integrated combination of intensive simulations and novel analytic theory that together provided a substantial advance in our understanding of these important molecular passing processes. With this kind of insight, in principle, porous nanoparticle systems can be optimized," said Evans.
The results were reported in Physical Review Letters.
More information: Physical Review Letters, journals.aps.org/prl/abstract/ … ysRevLett.113.038301
Journal information: Physical Review Letters
Provided by Ames Laboratory