New study revisits Miller-Urey experiment at the quantum level

For the first time, researchers have reproduced the results of the Miller-Urey experiment in a computer simulation, yielding new insight into the effect of electricity on the formation of life's building blocks at the quantum level.
In 1953, American chemist Stanley Miller had famously electrified a mixture of simple gas and water to simulate lightning and the atmosphere of early Earth. The revolutionary experiment—which yielded a brownish soup of amino acids—offered a simple potential scenario for the origin of life's building blocks. Miller's work gave birth to modern research on pre-biotic chemistry and the origins of life.
For the past 60 years, scientists have investigated other possible energy sources for the formation of life's building blocks, including ultra violet light, meteorite impacts, and deep sea hydrothermal vents.
In this new study, Antonino Marco Saitta, of the Université Pierre et Marie Curie, Sorbonne, in Paris, France and his colleagues wanted to revisit Miller's result with electric fields, but from a quantum perspective.
Saitta and study co-author Franz Saija, two theoretical physicists, had recently applied a new quantum model to study the effects of electric fields on water, which had never been done before. After coming across a documentary on Miller's work, they wondered whether the quantum approach might work for the famous spark-discharge experiment.
The method would also allow them to follow individual atoms and molecules through space and time—and perhaps yield new insight into the role of electricity in Miller's work.
"The spirit of our work was to show that the electric field is part of it," Saitta said, "without necessarily involving lightning or a spark."
Their results are published this week in the scientific journal Proceedings of the National Academy of Sciences.
An alternate route
As in the original Miller experiment, Saitta and Saija subjected a mixture of molecules containing carbon, nitrogen, oxygen and hydrogen atoms to an electric field. As expected, the simulation yielded glycine, an amino acid that is one of the simplest building blocks for proteins, and one the most abundant products in the original Miller experiment.
But their approach also yielded some unexpected results. In particular, their model suggested that the formation of amino acids in the Miller scenario might have occurred via a more complex chemical pathway than previously thought.
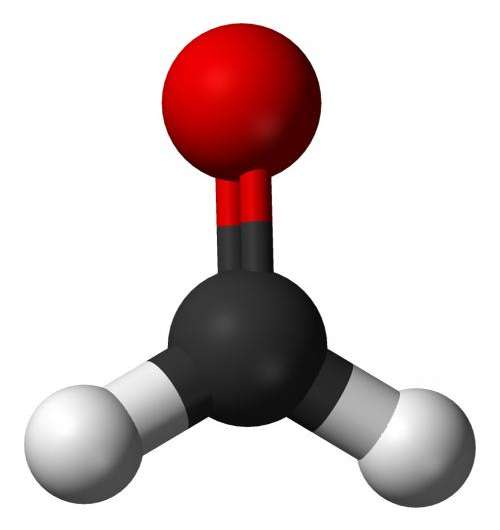
A typical intermediate in the formation of amino acids is the small molecule formaldehyde. But their simulation showed that when subjected to an electric field, the reaction favored a different intermediate, the molecule formamide.
It turns out, formamide could have not only played a crucial role in the formation of life's building blocks on Earth, but also elsewhere.
"We weren't looking for it, or expecting it," Saitta said. "We only learned after the fact, by reviewing the scientific literature, that it's an important clue in prebiotic chemistry."
For instance, formamide has recently been shown to be a key ingredient in making some of the building blocks of RNA, notably guanine, in the presence of ultra violet light.
Formamide has also recently been observed in space—notably in a comet and in a solar-type proto star. Earlier research has also shown that formamide can form when comets or asteroids impact the Earth.
"The possibility of new routes to make amino acids without a formaldehyde intermediate is novel and gaining ground, especially in extraterrestrial contexts," the authors wrote. "The presence of formamide might be a most telling fingerprint of abiotic terrestrial and extraterrestrial amino acids."
However, Jeff Bada, who was a graduate student of Miller's in the 1960s and spent his career working of the origin of life, remains skeptical about their results and theoretical approach.
"Their model might not meaningfully represent what happens in a solution," he says. "We know there's a lot of formaldehyde made in the spark discharge experiment. I don't think the formamide reaction would be significant in comparison to the traditional reaction."
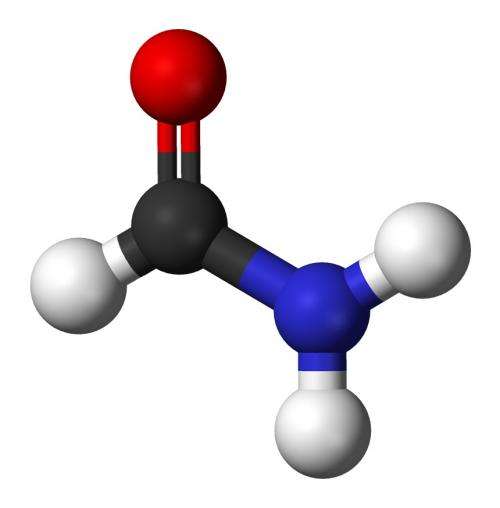
But Saitta points out that formamide is very unstable, so it may not last long enough to be observed in real Miller experiments. "In our simulation, formamide always formed spontaneously. And it was some sort of crucible—it would either break up into water and hydrogen cyanide, or combine with other molecules and form the amino acid glycine."
Life's origin–on the rocks?
Another key insight from their study is that the formation of some of life's building blocks may have occurred on mineral surfaces, since most have strong natural electric fields.
"The electric field of mineral surfaces can be easily 10 or 20 times stronger than the one in our study," Saitta said. "The problem is that it only acts on a very short range. So to feel the effects, molecules would have to be very close to the surface."
"I think that this work is of great significance," said François Guyot, a geochemist at the French Museum of Natural History.
"Regarding the mineral surfaces, strong electric fields undoubtedly exist at their immediate proximity. And because of their strong role on the reactivity of organic molecules, they might enhance the formation of more complex molecules by a mechanism distinct from the geometrical concentration of reactive species, a mechanisms often proposed when mineral surfaces are invoked for explaining the formation of the first biomolecules."
One of the leading hypotheses in the field of life's origin suggests that important prebiotic reactions may have occurred on mineral surfaces. But so far scientists don't fully understand the mechanism behind it.
"Nobody has really looked at electric fields on mineral surfaces," Saitta said. "My feeling is that there's probably something to explore there."
More information: — Paper: Antonino Marco Saitta and Franz Saija. "Miller experiments in atomistic computer simulations." PNAS 2014 ; published ahead of print September 8, 2014, DOI: 10.1073/pnas.1402894111
— Follow Johnny Bontemps on Twitter
Journal information: Proceedings of the National Academy of Sciences
Source: Astrobio.net
This story is republished courtesy of NASA's Astrobiology Magazine. Explore the Earth and beyond at www.astrobio.net .





















