To fight malaria, we now have genetic weapons that can track and kill

Every year malaria kills more than 600,000, which is a little less than the population of Bhutan. There are some simple solutions to control the disease, but keeping the numbers of mosquitoes with malarial parasites down remains a challenge.
The problem is that current control measures can wane in effectiveness as mosquitoes adapt. Discovering new ways to reduce mosquito numbers is therefore a high priority. An area where such advances are being made is genetics, and two recent studies are good examples of things to come.
Sex talk
Reducing the number of female mosquitoes, which are the transmitters of the malarial parasite, can drastically reduce the spread of the disease. In a study published in Nature Communications, researchers at Imperial College London are able to do that by using an enzyme that affects DNA.
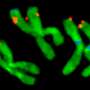
Their laboratory results show that, within a few generations, whole colonies of mosquitoes can disappear when they use this enzyme. The enzyme selectively shreds the DNA of female mosquito, affecting those bits involved in reproduction (in particular, the X chromosome). Its offspring then are nearly all male, leading to the collapse of the whole population.
Modifying the enzyme to damage the specific DNA took some time, and relied upon previous results that were initially obtained by curiosity-driven work. However, this new and successful application in the lab means field studies can begin soon.
Sleeping sickness in cattle, which is caused by tsetse flies, was eradicated from Zanzibar by the release of sterile male tsetse flies. But this was a costly and logistically demanding exercise that needed the saturation of a large area with nearly 10m sterile male flies produced by radiation treatment. The enzyme treatment might prove more cost effective, even though this is a different mosquito vector. It also gives more support to those in favour of genetic modification.
Genetic barcodes
Resistance to malarial drug patterns vary by geography. It would prove very useful if researchers are able to pinpoint the origin of a particular malarial strain, and researchers at the London School of Tropical Hygiene and Medicine have developed such a tool using genetic "barcodes".
To do this they analysed the genomes of more than 700 parasites found in Africa, Southeast Asia and Latin America. Their report, also published in Nature Communications, found that most of the genome wasn't useful, but the bits of DNA in the mitochondria (the cell's powerhouse) and the apicoplast (a remnant of the plant cell found in the parasites) gave enough unique information to create a barcoding system.
The upshot of this quirk is that the barcoding system should last and get better as more genetic data is collected. This is because these bits of the DNA won't change much. They are passed down from mother to kids without recombining, unlike the rest of the DNA.
It is not enough to develop a new drug to replace a failing one, or discover another insecticide in the face of resistance in mosquitoes. Such imaginative technologies to reduce the enormous global footprint of malaria are needed. With genetic tools, we have taken the fight against malaria to the molecular level.
Journal information: Nature Communications
Provided by The Conversation
This story is published courtesy of The Conversation (under Creative Commons-Attribution/No derivatives).
![]()