'Onion' vesicles for drug delivery developed
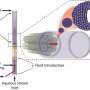
One of the defining features of cells is their membranes. Each cell's repository of DNA and protein-making machinery must be kept stable and secure from invaders and toxins. Scientists have attempted to replicate these properties, but, despite decades of research, even the most basic membrane structures, known as vesicles, still face many problems when made in the lab. They are difficult to make at consistent sizes and lack the stability of their biological counterparts.
Now, University of Pennsylvania researchers have shown that a certain kind of dendrimer, a molecule that features tree-like branches, offers a simple way of creating vesicles and tailoring their diameter and thickness. Moreover, these dendrimer-based vesicles self-assemble with concentric layers of membranes, much like an onion.
By altering the concentration of the dendrimers suspended within, the researchers have shown that they can control the number of layers, and thus the diameter of the vesicle, when the solution is injected in water. Such a structure opens up possibilities of releasing drugs over longer periods of time, with a new dose in each layer, or even putting a cocktail of drugs in different layers so each is released in sequence.
The study was led by professor Virgil Percec, of the Department of Chemistry in Penn's School of Arts & Sciences. Also contributing to the study were members of Percec's lab, Shaodong Zhang, Hao-Jan Sun, Andrew D. Hughes, Ralph-Olivier Moussodia and Annabelle Bertin, as well as professor Paul Heiney of the Department of Physics and Astronomy. They collaborated with researchers at the University of Delaware and Temple University.
Their study was published in Proceedings of the National Academy of Sciences.
Cell membranes are made of two layers of molecules, each of which has a head that is attracted to water and a tail that is repelled by it. These bilayer membranes self-assemble so that the hydrophilic heads of the molecules of both layers are on the exterior, facing the water that is in the cell's environment as well as the water encapsulated inside.
For decades, scientists have been trying to replicate the most basic form of this arrangement, known as a vesicle, in the lab. Stripping out the additional proteins and sugars that cells naturally have in their membranes leaves just a double-walled bubble that can be stuffed with drugs or other useful content.
"The problem," Percec said, "is that once you remove the proteins and the other elements of a real biological membrane, they are unstable and don't last for a long time. It's also hard to control their permeability and their polydispersity, which is how close together in size they are. The methodologies for producing them are also complicated and expensive."
Research in this field has thus been focused on finding new chemistries to replace the fatty molecules that normally make up a vesicle's bilayer.
The Percec group's breakthrough came in 2010, when they started making vesicles using a class of molecules called amphiphilic Janus dendrimers.
Like the Roman god Janus, these molecules have two faces. Each face has tree-like branches instead of the head and tail found in the molecules that make up biological membranes found in nature. But like those molecules, these dendrimers are amphiphilic, meaning that one face's branches is hydrophilic and the other is hydrophobic.
In 2010, Percec and his colleagues found the smallest possible amphiphilic Janus dendrimer. Dissolving those molecules in an alcohol solution and injecting them into water, the researchers found that they formed stable, evenly sized vesicles.
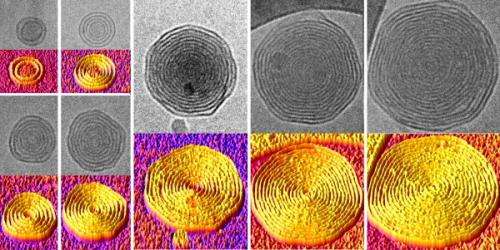
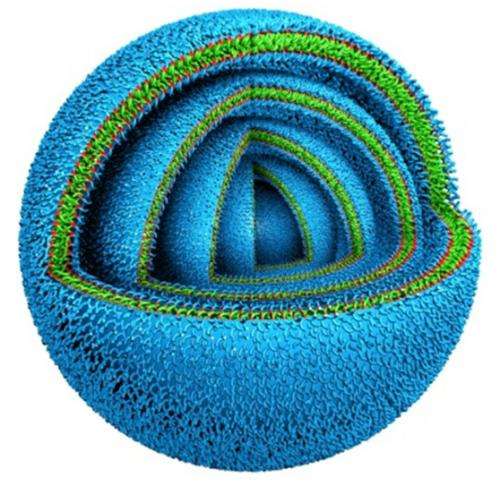
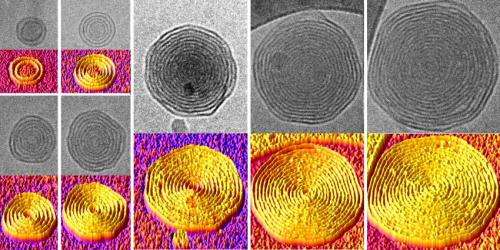
Not all cells are content with just a single bilayer, however. Some biological systems, such as gram-negative bacteria and the myelin sheaths that cover nerves, have multiple concentric bilayers. Having a model system with that arrangement could provide some fundamental insights to these real-world systems, and the added stability of extra layers of padding would be a useful trait in clinical applications. However, methods for producing vesicles with multiple bilayers remained elusive.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
"The only way it has been achieved in the past was through a complicated mechanical process, which was a dead end," Percec said. "This was not a viable option for mass-producing multilayered vesicles, but, with our library of amphiphilic Janus dendrimers, we were lucky to find some molecules that have in their chemicals instructions needed to self-assemble into these very beautiful structures."
By testing different dendrimers with different organic solvents, the research team found they could produce these onion-like vesicles and control the number of layers they contained. By changing the concentration of the dendrimers in the solvent, they could produce vesicles with as many as 20 layers when that solution was introduced to water. And because the layers are consistently spaced, the team could control the overall size of the vesicles by predicting the number of layers they could contain.
To actually see the multilayered structure of their vesicles, the researchers used a technique known as cryogenic transmission electron microscopy, or CryoTEM. This technique can take pictures of objects at the nanoscale floating in aqueous solution. To keep the fluid-floating vesicles in frame, the team flash-froze the sample, locking them in amorphous ice that was free of damaging ice crystals.
With the vesicles characterized, a host of clinical applications are possible. One of the more enticing is encapsulating drugs in these vesicles. Many drugs are not water-soluble, so they need to be packaged with some other chemistry to allow them to flow through the bloodstream. The additional stability of multiple bilayers makes these onion vesicles an attractive option, and their unique structure opens the door to next generation nanomedicine.
"If you want to deliver a single drug over the course of 20 days," Perce said, "you could think about putting one dose of the drug in each layer and have it released over time. Or you might put one drug in the first layer, another drug in the second and so on. Being able to control the diameter of the vesicles may also have clinical uses; target cells might only accept vesicles of a certain size."
More information: Self-assembly of amphiphilic Janus dendrimers into uniform onion-like dendrimersomes with predictable size and number of bilayers, www.pnas.org/content/early/201 … /1402858111.abstract
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Pennsylvania