April 28, 2014 feature
Room to move: Tissue growth controlled by cell cycle response to spatial and mechanical constraints
(Phys.org) —One of the most important factors in tissue formation is the control of cell proliferation. While the fact that cells undergo a range of spatial and mechanical constraints, the ways the resulting mechanical feedback may affect cell cycle progression – and thus tissue cell proliferation pattern – has not been fully understood. Recently, however, scientists at University of California, Santa Barbara, European Molecular Biology Laboratory, Heidelberg, Germany, and Stanford University studied a mammalian model epithelium's response to experimentally applied forces, finding a mechanosensitive checkpoint that controls cell cycle progression in response to spatial constraints. The study also showed that stretching the tissue results in fast cell cycle reactivation, whereas compression rapidly leads to cell cycle arrest – with cells having no memory of past constraints. This allowed them to develop a biophysical model that predicts tissue growth in response to environment changes in spatial constraints. The researchers say their findings suggest that this regulatory response may well maintain tissue integrity and control developmental and regenerative tissue growth. They conclude that this cellular memory-free adaptation to available space may coordinate cell proliferation and maintain tissue homeostasis.
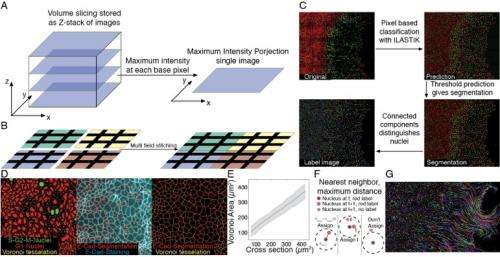
Dr. Sebastian J. Streichan discussed the paper that he and his co-authors published in Proceedings of the National Academy of Sciences. Regarding probing the impact of mechanical tissue perturbations on cell cycle progression, Streichan tells Phys.org that a central challenge is long-term live imaging of cultured cells. "On the one hand, the conditions have to be sterile over weeks not to contaminate the sample, and on the other hand keeping phototoxicity at a minimal level while having a reasonably high observation frequency. In addition, when dealing with cell cycle dynamics tissues subject to acute changes in boundary conditions, it's critical to develop a boundary release assay that does not involve wounding the tissue, as previous research1 has shown – for example, a scratch wound assay can trigger chemical signals – and the same work also showed that growing cells against a removable barrier prevents this." However, Streichan notes that tissue stretching and compression "were the trickiest experiments we had to perform – there were many small problems to overcome. For example, we discovered that the cells are very sensitive to the material used to build the stretcher, and had to systematically screen for material that allows for stable culturing of tissues."
Streichan points out that using kinetic analysis to show that cells have no memory of past constraints also had its hurdles. "We had to learn how to automatically segment cells while allowing for little loss, so that simple tracking methods could be applied – but the use of simple tracking methods introduced another challenge for microscopy, in which we had to be able to image frequently enough to reliably identify the nearest neighbor of cells, even in dynamic regions of the tissue." Moreover, he adds, formulating a biophysical model that predicts tissue growth in response to changes in spatial constraints in the environment is a slow process that involves the accumulation of many small observations. "The kind of cellular growth rule we should implement turned out to be important. For example," he illustrates," are cells an elastic material that is always growing, or is there some plastic growth control?"
In addressing these challenges, Streichan says that "our key insight, even though it has been theoretically predicted, was the direct observation that the cell cycle in tissues can be adjusted through mechanics – especially since the ongoing debate on size dependence of cell cycle regulation in single cells made us skeptical. Using a live readout of the cell cycle in combination with quantitative analysis, tissue stretching and boundary release experiments allowed us to construct a simplified picture of the decade-old puzzle of contact inhibition of proliferation."
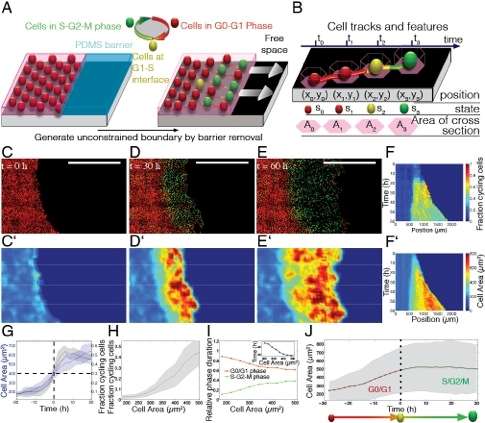
In the paper, the scientists detail the ways in which cells experience various spatial and mechanical constraints depending on their environmental context in the body. "During development, cells in tissues proliferate, changing the space available to individual members," Streichan explains. "Cells often stop proliferating when density reaches a critical limit" (This phenomenon is known as density-dependent contact proliferation inhibition.) "In tissues, cells are mechanically coupled through adhesive cell-cell contacts – and as we exercise, cells in our body will be subject to stresses. In another situation, during regeneration after a tissue is wounded, spatial constraints imposed by neighboring cells are lifted at the newly-generated boundary, allowing cells to reactivate their cell cycle."
Another finding presented in the paper is that the ability of tissues to support cell cycle progression adapts to the available space through a memory-free control mechanism that may coordinate proliferation patterns to maintain tissue homeostasis. "We used a dynamic cell cycle marker – specifically, a Fluorescent Ubiquitination-based Cell Cycle Indicator, or FUCCI – to distinguish cells that committed to the cell cycle from cells that are not yet in the cell cycle," Streichan tells Phys.org. (Ubiquitination regulates degradation of cellular proteins by the ubiquitin-proteasome system, controlling a protein's half-life and expression levels.) "This allowed us to identify a monotonic dependence of cell cycle progression on the space covered by a cell." In so doing, the researchers found that the more space a cell was given, the more often they would find it committed to the cell cycle.
"This dependence," he continues, "was also observed after stretching or compression of tissues, which made us wonder if there is some kind of hysteresis in cell cycle progression. Using pharmacological cell cycle inhibitors, we could block cells from entering the cell cycle, while keeping the tissue stretched." As a result, he notes, the cells experienced altered spatial constraints, which should favor cell cycle progression; drug washout releases the block, and the resulting simultaneous relaxation of the tissue reinstalls spatial constraints that disfavor cell cycle progression.
"If the decision for cell cycle progression depended on previously available space, we would expect to see cells entering the cell cycle, which we did not," he points out. "We therefore interpreted these results as a memory-free cell cycle regulation through spatial constraints. Moreover," he adds, "cells needed to be stretched for a minimal amount of time in order to enter the cell cycle. Together, such a control mechanism may be used to allow tissues to respond elastically to a transient stretch and plastically adapt to sustained stretch."
The scientists propose that controlled tissue growth in many developmental and tumor invasion contexts is mediated by a mechanosensitive checkpoint that monitors spatial constraints to control cell cycle progression at the G1/S boundary. (Mechanosensitivity is a cell's specific response to mechanical stimulation. The G1/S boundary is a stage in the cell cycle at the interface between the G1 phase and the S phase, beyond which the cell is committed to dividing.) "Our data show that cells sense spatial constraints in their local environment and accordingly progress in the cell cycle," Streichan explains, "and the collective motion we observe in our boundary release experiments has intriguing parallels to the behavior in invasive tissues. On the other hand," he points out, "tissues know how to repair wounds and cover the wound site with the right number of cells, even though it's difficult to imagine how they can determine wound size. Similarly, during development, organs are built roughly from the same number of cells, and reach roughly the same size, suggesting that a mechanosensitive checkpoint may also be at work in vivo." Streichan adds that investigating the molecular mechanism of cell growth regulation at the G1/S boundary will help them to map this process in more detail.
The scientists also propose that differential responses from cells and tissues might result from mechanical coupling between cells in tissues via their adhesive cell–cell contacts. "Cell coupling is achieved through so-called adherens junctions that contain many proteins – some of which, such as cadherin, interact across cells," Streichan says. (In an epithelium, the cell surfaces are crowded with cadherins, which can bind to the cadherin of neighboring cells.) "At the same time, within the cell these adherens junctions are coupled to the actin cytoskeleton, which can pull on these adherens junctions. This may trigger a signal in some of the constituent proteins, eventually activating a signaling cascade. Research in the field identified a heterogeneous stress landscape in tissues, suggesting that such mechanical signaling mechanisms may lead to heterogeneous responses of cells in tissues."
When asked if their observation that stretching the tissue results in fast cell cycle reactivation and compression rapidly leads to cell cycle arrest might be accounted for by torsional strain as a derivative of changes in spatial constraints, Streichan says that based on their measurements, it is hard to distinguish whether cells measure stress or strain. "The difficulty is also in the fact that we don't know the stress/strain constitutive equation for tissues – and while some experiments indicate it is stress, the final answer remains to be found." Streichan also acknowledges that other open questions remain, one being whether mechanical constraints control cell cycle progression in growing tissues. "Having analyzed this process in a model epithelium in a Petri dish, the next step would be to study this phenomenon using in vivo systems, such as the wing disc of Drosophila melanogaster larvae and repeat our analysis."
If the biomechanical model developed in the study might lead to translational or clinical applications in wound healing, Streichan predicts, it will be a long process. "However, there are intriguing parallels. I recently learnt from a fellow researcher about eye treatments – similar to our boundary release assay – in which surgeons debrides unhealthy corneal cells, and fresh cells then occupy the resulting free space relatively quickly. Personally, I find the analogy to growing skin – for example, during pregnancy – Interesting. Perhaps one may find a way to even overcome generation of stretch marks, based on a better understanding of the mechanical control of cell cycle regulation. However," he cautions, "general wounds, such as a deep cut, are much more complex and will involve many other factors that we couldn't consider in our experimental setting."
In terms of tissue development and disease, Streichan notes that although the scientists verified mechanotransduction (the cellular translation of mechanical signals into biochemical signals) in tissue culture only, there is reason to believe that similar mechanisms might act in vivo during development. "Using the analogy to growing skin again," Streichan illustrates, "such a mechanical regulation would ensure the 'right fit' of skin around our body. Moreover, in the body, organs are lined by tissues, which also must reach the right size. Loosely speaking, cancer is uncontrolled growth – so given that mechanics is a growth regulator, it will be important to understand the molecular mechanism, which may reveal novel oncogenes."
Moving forward, says Streichan, the researchers would like to understand the molecular mechanism of mechanical feedback as a growth regulator. "For this," he notes, "we're generating a candidate screen, with which we hope to identify the network of proteins involved in its regulation." Moreover, Streichan concludes, there are other areas of research that might benefit from their study. "Our work may be helpful for certain medical applications, and perhaps support our understanding of contact inhibition of proliferation in developmental contexts. It also stresses the importance to study mechanotransduction."
More information: Spatial constraints control cell proliferation in tissues, Proceedings of the National Academy of Sciences, vol. 111 no. 15, 5586-5591 (2014), doi:10.1073/pnas.1323016111
Related:
1Role of boundary conditions in an experimental model of epithelial wound healing, American Journal of Physiology – Cell Physiology, 291(1):C68–C75 (2006), doi:10.1152/ajpcell.00411.2005
Journal information: Proceedings of the National Academy of Sciences
© 2014 Phys.org