Researchers show stem cell fate depends on 'grip'
The field of regenerative medicine holds great promise, propelled by greater understanding of how stem cells differentiate themselves into many of the body's different cell types. But clinical applications in the field have been slow to materialize, partially owing to difficulties in replicating the conditions these cells naturally experience.
A team of researchers from the University of Pennsylvania has generated new insight on how a stem cell's environment influences what type of cell a stem cell will become. They have shown that whether human mesenchymal stem cells turn into fat or bone cells depends partially on how well they can "grip" the material they are growing in.
The research was conducted by graduate student Sudhir Khetan and associate professor Jason Burdick, along with professor Christopher Chen, all of the School of Engineering and Applied Science's Department of Bioengineering. Others involved in the study include Murat Guvendiren, Wesley Legant and Daniel Cohen.
Their study was published in the journal Nature Materials.
Much research has been done on how stem cells grow on two-dimensional substrates, but comparatively little work has been done in three dimensions. Three-dimensional environments, or matrices, for stems cells have mostly been treated as simple scaffolding, rather than as a signal that influences the cells' development.
Burdick and his colleagues were interested in how these three-dimensional matrices impact mechanotransduction, which is how the cell takes information about its physical environment and translates that to chemical signaling.
"We're trying to understand how material signals can dictate stem cell response," Burdick said. "Rather than considering the material as an inert structure, it's really guiding stem cell fate and differentiation—what kind of cells they will turn into."
The mesenchymal stem cells the researchers studied are found in bone marrow and can develop into several cell types: osteoblasts, which are found in bone; chondrocytes, which are found in cartilage; and adipocytes, which are found in fat.
The researchers cultured them in water-swollen polymer networks known as hydrogels, which share some similarities with the environments stem cells naturally grow in. These materials are generally soft and flexible—contact lenses, for example, are a type of hydrogel—but can vary in density and stiffness depending on the type and quantity of the bonds between the polymers. In this case, the researchers used covalently cross-linked gels, which contain irreversible chemical bonds.
When seeded on top of two-dimensional covalently cross-linked gels, mesenchymal stem cells spread and pulled on the material differently depending on how stiff it was. Critically, the mechanics guide cell fate, or the type of cells they differentiate it into. A softer environment would produce more fat-like cells and a stiffer environment, where the cells can pull on the gel harder, would produce more bone-like cells.
However, when the researchers put mesenchymal stem cells inside three-dimensional hydrogels of varying stiffness, they didn't see these kinds of changes.
"In most covalently cross-linked gels, the cells can't spread into the matrix because they can't degrade the bonds—they all become fat cells," Burdick said. "That tells us that in 3D covalent gels the cells don't translate the mechanical information the same way they do in a 2D system."
To test this, the researchers changed the chemistry of their hydrogels so that the polymer chains were connected by a peptide that the cells could naturally degrade. They hypothesized that, as the cells spread, they would be able to get a better grip on their surrounding environment and thus be more likely to turn into bone-like cells.
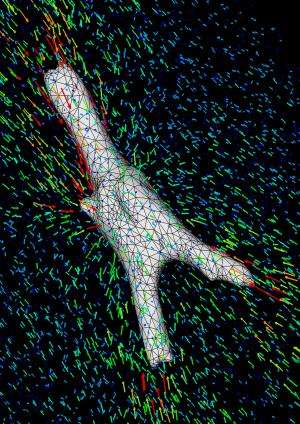
In order to determine how well the cells were pulling on their environment, the researchers used a technique developed by Chen's lab called 3D traction force microscopy. This technique involves seeding the gel with microscopic beads, then tracking their location before and after a cell is removed.
"Because the gel is elastic and will relax back into its original position when you remove the cells," Chen said, "you can quantify how much the cells are pulling on the gel based on how much and which way it springs back after the cell is removed."
The results showed that the stem cells' differentiation into bone-like cells was aided by their ability to better anchor themselves into the growth environment.
"With our original experiment, we observed that the cells essentially didn't pull on the gel. They adhered to it and were viable, but we did not see bead displacement. They couldn't get a grip," Burdick said. "When we put the cells into a gel where they could degrade the bonds, we saw them spread into the matrix and deform it, displacing the beads."
As an additional test, the researchers synthesized another hydrogel. This one had the same covalent bonds that the stem cells could naturally degrade and spread through but also another type of bond that could form when exposed to light. They let the stem cells spread as before, but at the point the cells would begin to differentiate—about a week after they were first encapsulated—the researchers further "set" the gel by exposing it to light, forming new bonds the cells couldn't degrade.
"When we introduced these cross-links so they could no longer degrade the matrix, we saw an increase toward fat-like cells, even after letting them spread," Burdick said. "This further supports the idea that continuous degradation is needed for the cells to sense the material properties of their environment and transduce that into differentiation signals."
Burdick and his colleagues see these results as helping develop a better fundamental understanding of how to engineer tissues using stem cells.
"This is a model system for showing how the microenvironment can influence the fate of the cells," Burdick said.
More information: www.nature.com/nmat/journal/va … t/full/nmat3586.html
Journal information: Nature Materials
Provided by University of Pennsylvania