Hybrid 'Janus' nanoparticles made from gold and titania have high catalytic activity and extraordinary durability
As recently as twenty-five years ago, chemists considered gold to be one of the most inert metallic elements, until the discovery that nanoscale-sized dispersions of gold had high catalytic activity forced a re-think of old principles. Researchers soon found that gold nanoparticles could promote many industrially important reactions, such as the removal of harmful carbon monoxide gas from emission streams. Whilst the benefits of nanoscale gold are well-attested, preparing the material in a durable and reusable form remains a significant challenge that limits its uptake by manufacturers.
Work by the teams of Ming-Yong Han of the Institute of Materials Research and Engineering and Yong-Wei Zhang from the Institute of High Performance Computing both at A*STAR has revealed that the stability of gold nanoparticle catalysts can be enhanced by coating them with protective titania (TiO2) layers. Conceived by co-author Zhi Wei Seh, an A*STAR National Science Scholar, this new technique produces so-called Janus nanostructures that retain nearly all the catalytic activity of bare gold nanoparticles without suffering from irreversible aggregation that diminishes the reactivity of the latter.
Named after the twin-faced Roman god of beginnings and transitions, Janus nanostructures join two or more equal-sized components together through very small junctions — an arrangement that maximizes the active surface area of each substance. The beneficial effects of pairing gold nanoparticles with titania is well known, but until the work by A*STAR researchers, a detailed understanding of the mechanism by which these two species fuse together had proved elusive.
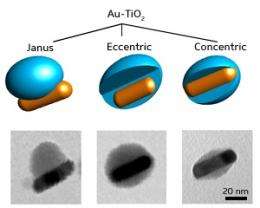
Han and co-workers used an unconventional chelating compound called titanium diisopropoxide bis(acetylacetonate) to nucleate the growth of TiO2 onto gold at extremely slow rates. By carefully controlling the addition of this reagent to rod- and spherical-shaped gold nanoparticles, the researchers observed three distinct nanostructures (see image): a Janus geometry; a partially encapsulating ‘eccentric’ geometry; and a ‘concentric’ core-shell arrangement.
Catalytic experiments revealed that the reactivity and durability of gold-titania Janus structures have unique advantages over other nanoparticles. Due to the exposed nature of their gold surfaces, the former catalyze the reduction of the molecule 4-nitro phenol at much faster rates than eccentric and concentric nanoparticles whose gold surfaces are more confined. Furthermore, the protective TiO2 coating of the hybrid catalysts allowed them to be reused repeatedly with little loss of activity. In contrast, bare gold nanoparticles agglomerated into un-reactive clumps after just five usage cycles.
Futher theoretical investigations by the team revealed that the formation of Janus nanostructures as the energetically stable species is promoted by the addition of smaller volumes of the titania precursor — a finding that may help the researchers generate other metal–oxide hybrids for catalytic applications in the near future.
More information: Seh, Z. W. et al. Anisotropic growth of titania onto various gold nanostructures: Synthesis, theoretical understanding, and optimization for catalysis. Angewandte Chemie International Edition 50, 10140–10143 (2011).

















