New super strong alloy discovered
(PhysOrg.com) -- International team of researchers has discovered a new super-strength light alloy and had their key findings published in Nature Communications.
A North Carolina State University researcher and colleagues have figured out a way to make an aluminum alloy, or a mixture of aluminum and other elements, just as strong as steel.
That's important, says Dr. Yuntian Zhu, professor of materials science and the NC State researcher involved in the project, because the search for ever lighter - yet stronger - materials is crucial to devising everything from more fuel-efficient cars to safer airplanes.
In a paper published in the journal Nature Communications, Zhu and his colleagues describe the new nanoscale architecture within aluminum alloys that have unprecedented strength but also reasonable plasticity to stretch and not break under stress. Perhaps even more importantly, the technique of creating these nanostructures can be used on many different types of metals.
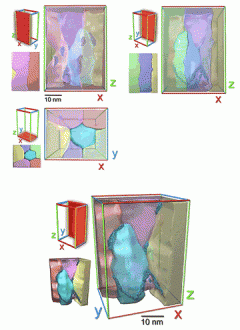
Zhu says the aluminum alloys have unique structural elements that, when combined to form a hierarchical structure at several nanoscale levels, make them super-strong and ductile.
The aluminum alloys have small building blocks, called "grains," that are thousands of times smaller than the width of a human hair. Each grain is a tiny crystal less than 100 nanometers in size. Bigger is not better in materials, Zhu says, as smaller grains result in stronger materials.
Zhu also says the aluminum alloys have a number of different types of crystal "defects." Nanocrystals with defects are stronger than perfect crystals.
The unexpectedly high level of strengthening appears to be due to two factors. Firstly, the way that the alloying elements are arranged within the grains is thought to increase the dislocation-storage capacity of the alloy. Secondly, the clustering of elements between the grains could limit nanocrystal growth, increase the cohesion of the grains, and resist embrittlement and defect generation.
Now, Zhu plans on working on strengthening magnesium, a metal that is even lighter than aluminum. He's collaborating with the Department of Defense on a project to make magnesium alloys strong enough to be used as body armor for soldiers.
Zhu's colleagues on the Nature Communications paper are affiliated with the University of Sydney in Australia; the University of California, Davis; and Ufa State Aviation Technical University in Russia.
More information: "Nanostructural hierarchy increases the strength of aluminium alloys" Authors: Yuntian Zhu, North Carolina State University; Peter Liddicoat, Simon P. Ringer and Xiao-Zhou Liao, University of Sydney; Yonghao Zhao and Enrique J. Lavernia, University of California, Davis; Maxim Y. Murashkin and Rusian Z. Valiev, Ufa State Aviation Technical University. Published: Sept. 7, 2010, in Nature Communications.
Provided by North Carolina State University