September 2, 2010 feature
Scientists investigate how ice melts below freezing due to nanowire's pressure
(PhysOrg.com) -- The many ways in which water differs from other molecules is both a scientific curiosity and an important factor in shaping the Earth. Among water's unique properties are that it expands when it freezes, it boils and freezes at higher temperatures than expected for a compound with its molecular structure, and it has the ability to absorb large amounts of heat without getting hot. In a recent study, scientists have investigated another unique phenomenon of water called regelation, which occurs when frozen water - or ice - melts under high pressures, even if the temperature is below freezing. Once the pressure is lifted, the water refreezes.
The scientists, Teemu Hynninen from the Tampere University of Technology and the Aalto University School of Science and Technology in Finland, as well as coauthors from Finland, Canada, and the US, have performed simulations of the pressure-induced melting of ice by cutting a block of ice with a nanowire. The experiment is similar to a classic experiment performed over a century ago in which scientists demonstrated that a thin, weighted wire could slowly pass through an ice block due to the pressure it exerts on the ice. In the new study, the scientists simulated a nanowire, whose diameter was on the scale of the water molecules, to investigate the molecular-level mechanisms responsible for regelation.
“We decided to study the wire-cutting experiment because it has historical signifigance, it is conceptually simple, and it hadn't been studied on the microscopic scale,” Hynninen told PhysOrg.com. “In this case, we have a wire moving through ice (instead of sliding on it like in normal friction), and the motion is governed by complicated interplay between the wire and the liquid and solid phases of water.”
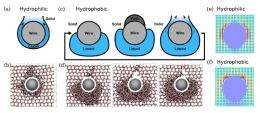
In the simulations, the nanowire is depicted as a string of beads that interact with the H2O molecules. When these beads exert a pressure on the H2O molecules, they break the hydrogen bonds between the oxygen atom of one molecule and the hydrogen atom of another molecule. This breaks the ice lattice structure and transforms the solid ice into liquid water, allowing the nanowire to move through the water, even though the temperature is below freezing.
As the scientists explain, the critical point at which the nanowire melts the ice can be thought of as a depinning transition, which generally refers to the yield point of a solid under stress. The simulations showed that this critical point depends on the type of wire used. The scientists compared two types of nanowires - hydrophilic, i.e., a wire whose surface attracts water, and hydrophobic, i.e., a wire whose surface repels water - and found that the wires moved differently through the ice after the depinning transition.
The difference is due to the nanowires' different structural properties, which causes them to interact differently with the surrounding water molecules. The hydrophilic nanowire exhibits a continuous transition at depinning, where the water can easily flow around it, allowing for smooth movement through the ice block. The hydrophobic wire, on the other hand, exhibits a discontinuous transition, where the water builds up thickly on one side of the nanowire. This wire can only move once the water layer builds up high enough so that it reaches the top of the wire, at which point it flows down the other side into a small void, allowing the nanowire to cut through the ice.
As the scientists explain, this behavior is somewhat counterintuitive, since a hydrophobic wire might be expected to move more quickly through water than a hydrophilic one due to the latter's closer water contact and increased friction. However, in this case, the hydrophobic wire's resistance to water causes it to be partly obstructed by solid ice, giving it more resistance to movement than the hydrophilic wire.
“The physically most interesting result we found is that there is a clear difference between the hydrophobic and hydrophilic wires in the way they depin, i.e., how they start moving as the driving force increases,” Hynninen said. “Although the nanowire we use here is a very simple object, our work shows that small structures in contact with water and ice may exhibit unexpected, complicated behavior.”
This qualitative understanding and the additional detail provided by the simulations demonstrates how the pressure-induced melting of ice depends on the wetting properties of the object - in this case, the nanowire - applying the pressure. The molecular-scale mechanisms that explain this unique property of water could allow scientists to better understand how the pressure-induced melting and freezing of water has helped shape the Earth. For example, the phenomenon of regelation acts in systems such as glaciers, allowing pressurized ice sheets to flow around obstacles.
“This study is basic research, not aimed to solve any particular problem,” Hynninen said. “Having said that, the work is part of a bigger project on the friction of ice at the nanoscale, and there the applications are more obvious. The slipperiness of ice and wet surfaces are significant issues in, e.g., transportation, and better understanding of these phenomena at the microscopic level could help design coatings or microstructures with desired frictional properties for tires, shoes, sports equipment, etc.”
More information: Teemu Hynninen, et al. “Cutting Ice: Nanowire Regelation.” Physical Review Letters 105, 086102 (2010). DOI:10.1103/PhysRevLett.105.086102
Copyright 2010 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.
















