July 19, 2010 report
New catalyst for hydrogen fuel cells resists CO contamination
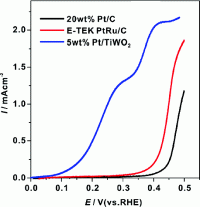
(PhysOrg.com) -- Hydrogen fuel cell vehicles promise faster refueling and the ability to travel longer distances before refueling than battery-powered cars, but they are susceptible to poisoning by carbon monoxide (CO). Now, scientists in the US and Japan have created new nanoparticles catalysts that enable hydrogen fuel cells to resist CO poisoning.
Hydrogen fuel cells use platinum electrocatalysts to combine hydrogen and oxygen to produce water and generate electricity. The problem is that the hydrogen is produced from sources such as gasoline, natural gas, or ethanol, and the process often introduces carbon monoxide into the gas. Even miniscule amounts of carbon monoxide in the hydrogen are sufficient to bind to the platinum catalysts and prevent them working. Scientists at Brookhaven National Lab in New York have recently found a platinum/ruthenium catalyst that blocks CO poisoning, but since this catalyst is extremely expensive, researchers have been seeing an alternative.
The new catalyst was developed by Professor Héctor Abruña and colleagues from Cornell University, the National Institute for Materials Science in Ibaraki, Japan, and the University of Pennsylvania. They began with the knowledge that tungsten alloys resist CO poisoning. Tungsten is not used in fuel cell electrodes because it is a poor electrical conductor, so Abruña and the team added tungsten to nanoparticles of titanium dioxide, which is a good electrical conductor. The result was titanium tungsten oxide nanoparticles, which they coated with platinum to make an electrode.
The researchers tested their nanoparticles catalysts with hydrogen contaminated with two percent carbon monoxide, and found performance was reduced by only five percent compared to 30 percent for ordinary catalysts.
Abruña said he is not sure how the new catalyst works, and much more testing is required, but he thinks a likely mechanism is that hydroxide (OH-) groups bind during the reaction to the titanium tungsten oxide near to the platinum, where they are close enough to the CO molecules to react and form CO2.
If the tests prove successful and the new catalyst can be made economically, it could spark renewed interest in using liquid fuels such as gasoline in cars to make the hydrogen required to power fuel cells. This in turn could enable fuel cells cars to have a longer range than those using gaseous hydrogen and those using gasoline conventionally.
The research paper was published in the Journal of the American Chemical Society.
More information: Highly Stable and CO-Tolerant Pt/Ti0.7W0.3O2 Electrocatalyst for Proton-Exchange Membrane Fuel Cells, J. Am. Chem. Soc., Article ASAP, Publication Date (Web): July 12, 2010. pubs.acs.org/doi/abs/10.1021/ja102931d
© 2010 PhysOrg.com

















