Chemists grow crystals with a twist -- and untwist
(PhysOrg.com) -- Chemists from New York University and Russia's St. Petersburg State University have created crystals that can twist and untwist, pointing to a much more varied process of crystal growth than previously thought. Their work, which appears in the latest issue of the Journal of the American Chemical Society, may explain some of the properties of high-polymers, which are used in clothing and liquid crystal displays, among other consumer products.
Crystal growth has traditionally been viewed as a collection of individual atoms, molecules, or small clusters adding to a larger block that remains in a fixed translational relationship to the rest.
But the NYU and St. Petersburg State University chemists discovered a wholly new phenomenon for growth— a crystal that continually changes its shape as it grows.
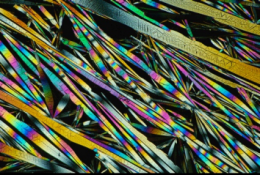
To do this, the researchers focused on crystals from hippuric acid—a derivative of the amino acid glycine. As molecules were added to the end of fine crystalline needles, stresses built up at the tips of the crystals and resulted in a helical twist—much like DNA's double helix. The process was reversed when crystals thickened from the opposite end of the growing tip—that is, the crystals stiffened, thereby undoing the twisted formations. This is because the elasticity of the crystals decreases as they become thicker, thus "squeezing out" the deformations that were induced at the growing tip.
"This competition between twisting and untwisting creates needles with a rainbow of colors, which is a characteristic of tightly wound helices, as well as ribbons that have become completely untwisted," said Bart Kahr, one of the study's co-authors and a professor in NYU's Department of Chemistry, explaining the crystals' appearance. "This is a very strange and new perspective on crystal growth."
"This dynamic has not been observed before and points to a much more active process of crystal growth than we had anticipated," added Kahr, also part of NYU's Molecular Design Institute.
Provided by New York University
















