On the road to 'sweet' tires made with a more sustainable process

Motorists will be driving on the world's first "green" tires within the next five years, scientists predicted here today, thanks to a revolutionary new technology that produces a key tire ingredient from renewable feedstocks rather than petroleum-derived feedstocks. The technology, described at the 239th National Meeting of the American Chemical Society (ACS), stands to reduce the tire industry's reliance on crude oil -- seven gallons of which now go into each of the approximately one billion tires produced each year worldwide.
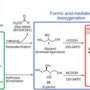
The new tires will be a "sweet" advance toward greener, more sustainable transportation in a quite literal sense, according to Joseph McAuliffe, Ph.D., who reported on the technology. The process can use sugars derived from sugar cane, corn, corn cobs, switchgrass or other biomass to produce the ingredient, a biochemical called isoprene, derived from renewable raw materials.
"An intensive search has been underway for years for alternative sources of isoprene, in particular those from renewable resources such as biomass," said McAuliffe. He is a staff scientist at Genencor, an industrial biotechnology company in Palo Alto, Calif. "One technical challenge has been the development of an efficient process for converting sugars into isoprene. One means by which we're addressing this challenge is by using a fermentation process based on a modified bacterial strain that is designed to convert carbohydrate feedstocks into BioIsoprene™ product."
The Goodyear Tire & Rubber Co. and Genencor, a division of Danisco A/S, have established a research collaboration to develop an integrated fermentation, recovery and purification system for producing BioIsoprene product from renewable raw materials. Genencor intends to commercialize the technology within the next five years.
In his ACS presentation, McAuliffe described how Genencor engineered bacteria to efficiently convert sugars to isoprene and how the smooth integration of fermentation and recovery processes promises to deliver a new route to this strategically important ingredient used to make synthetic rubber.
Goodyear and other large tire manufacturers use isoprene to produce synthetic rubber for use in tires to supplement use of natural rubber. Additionally, isoprene is used in a wide range of other industrial applications including, for example: use in other elastomers; block copolymers such as styrene-isoprene-styrene (SIS) used in hot melt adhesives in products such as diapers and feminine hygiene products; surgical gloves and other rubber-based products. Worldwide production of high purity isoprene derived today from petroleum-based feedstocks totals about 1.7 billion pounds. Goodyear, which manufactures 200 million tires annually, is one of the world's largest users of isoprene. The firm plans to supplement its use of petroleum-based isoprene with BioIsoprene product.
"This is an enormous market," McAuliffe said. "BioIsoprene™ product will serve as a renewable and cost-competitive alternative to isoprene. It's a material that can drive new markets, so I believe those numbers highlighting global consumption would grow if new material became available."
"We want to make biochemicals from renewable materials," McAuliffe said, "partially as a hedge against rising crude oil prices and much more so because this approach moves us to a more sustainable future."
Provided by American Chemical Society