Scientists find donut-shaped structure of enzyme involved in energy metabolism
If subway terminals didn't exist and people had to exit subway stations to switch subway lines, transit time would increase. People also may encounter distractions, such as grabbing a cup of coffee, instead of getting on the other line. Molecules also use "terminals" to save transit time during enzyme-catalyzed processes.
Using advanced X-radiation techniques, University of Missouri researchers were able to visualize one of these terminals inside of an enzyme that degrades proline, which is an amino acid that has a central role in metabolism. In humans, proline is important for suppression of cancer, cell death and oxidation. Understanding the structure of this enzyme will help scientists better understand how it functions and develop drugs that may inhibit its catalytic function.
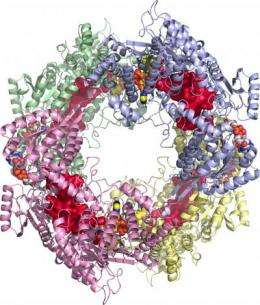
"This is an aesthetically interesting enzyme that resembles a donut-shaped ring," said John Tanner, professor in the Department of Chemistry and the Department of Biochemistry. "Hidden under the surface of the protein is a system of tunnels and rooms - like a subway system for molecules. The purpose of this system is to provide an interior passageway connecting the two catalytic sites of the enzyme. The movement of reactant molecules through this passageway is known as channeling, which makes enzymes efficient by isolating the reactants from other enzymatic reactions. Channeling potentially allows for decreased transit time between catalytic sites and protection from competing enzymatic reactions. The reactions occur without the reactants ever leaving the confines of the protein, which is efficient."
In the study, several proline-degrading proteins were screened for their ability to crystallize. A crystal is needed in order to perform X-ray diffraction experiments, which provide high resolution images of the protein's three-dimensional structure. Additional studies using small-angle X-ray scattering and centrifugation provided crucial information about the protein's donut shape. These techniques help researchers determine the structure and composition of the enzyme.
"The complementary methods of the X-ray crystallography, small-angle X-ray scattering, and centrifugation gave us a whole picture of the structure of the enzyme," Tanner said. "Knowing the structure of the enzyme helps us understand the function of the enzyme. Once we know an enzyme's structure, we can begin to interpret other important data, such as the enzyme's role in specific reactions, how its activity is controlled and how a drug could inhibit the enzyme."
The study, "Crystal structure of the bifunctional Proline utilization A flavoenzyme from Bradyrhizobium japonicum," was published in Proceedings of the National Academy of Sciences this month.
Provided by University of Missouri-Columbia


















