Energy Efficient Separations: Researchers Use Computational Modeling to Design Improved Membrane Technology
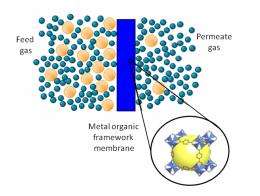
(PhysOrg.com) -- Computational modeling tools developed at the Georgia Institute of Technology could accelerate development of a new type of membrane technology that will boost the efficiency of energy-related gas separations. The tools will help researchers identify the best candidate materials for use in new metal-organic framework (MOF) membranes now under development.
MOF membranes offer an alternative to more energy intensive processes for separating gases such as carbon dioxide, methane, nitrogen and hydrogen. The technology has generated significant interest because of the broad range of crystalline structures that can be synthesized, but development of new MOF membranes is still at an early stage.
“Metal-organic framework membranes will be useful for doing large-scale energy-related separations in an efficient way. We are trying to accelerate their development to help move the world’s energy economy toward a more sustainable path,” said David Sholl, a professor in the Georgia Tech School of Chemical and Biomolecular Engineering. “A lot of chemists are interested in developing these metal-organic frameworks, and we hope to provide a new approach to designing the membranes.”
A publication on the use of atomically detailed calculations for designing metal-organic framework membranes was recently cited by ScienceWatch as its “fast-breaking paper in engineering” for February 2010. Details of the work were published in the journal Industrial Engineering Chemical Research in January 2009. The research was funded in part by the National Science Foundation.
Metal-organic framework materials are nanoporous crystals that combine metal-organic complexes with organic linkers to create highly porous frameworks. They offer advantages such as high surface area, porosity, low density and both thermal and mechanical stability - all important for separation membranes.
There are many possible material combinations that could be used in the membranes. By comparing such properties as binding strength and flow rates, the computational modeling could give researchers a way to rapidly identify the materials that will work best in high-volume industrial applications.
“The extra challenge with using metal-organic frameworks is that there are literally thousands of different materials that could be considered for use,” said Sholl, who is a Georgia Research Alliance eminent scholar in energy sustainability. “This is where computational modeling really helps. We are doing the materials screening problem computationally to guide us in attacking the actual fabrication problem experimentally.”
Sholl hopes the technique will narrow the list of candidate materials from thousands down to as few as 10. Researchers would then fabricate the membranes and test them in real-world conditions.
“If we were testing all of these in the lab without the computational guidance, it’s unlikely that we would ever choose the right material,” he said. “The biggest challenge for making a new membrane is that it really requires a lot of work to make a functioning device. Even if we know exactly what material to use, there is a very long development path.”
At Georgia Tech, Sholl’s modeling group is working with experimentalists such as Sankar Nair and Christopher Jones - both professors in the School of Chemical and Biomolecular Engineering - to produce prototype membranes for evaluation.
“The big push right now is to make some devices and get test data,” Sholl said. “In particular, we want to do this within a technology framework that we know can scale up to real-world industrial levels quickly.”
In addition to colleagues at Georgia Tech, the group is also working with industrial partners to help ensure that the membranes work in industrial conditions.
“If we can go from the idea in the academic lab to a serious field test within five years, that would be a real success,” said Sholl, who holds the Michael Tennenbaum Family Chair in the School of Chemical and Biomolecular Engineering. “We can’t afford for this to take 25 years because there is a need for this technology now.”
The new membrane technology could be used to address environmental issues such as removal of carbon dioxide from stack gases of coal-burning facilities in a cost-effective way. The technology could also make it economically attractive to use natural gas supplies that are contaminated with carbon dioxide, potentially expanding supplies of that fuel.
The researchers, including graduate student Seda Keskin, have modeled how the membrane technology would operate in separating methane from carbon dioxide, hydrogen from carbon dioxide, nitrogen from carbon dioxide, hydrogen from methane, nitrogen from hydrogen and methane from nitrogen.
“The common thread of this work is that we are interested in very large scale, large volume applications that can only be economical with very low energy input,” Sholl added.
Provided by Georgia Institute of Technology

















